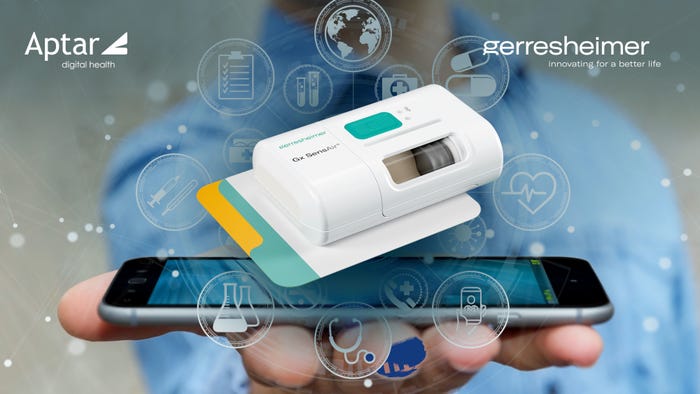
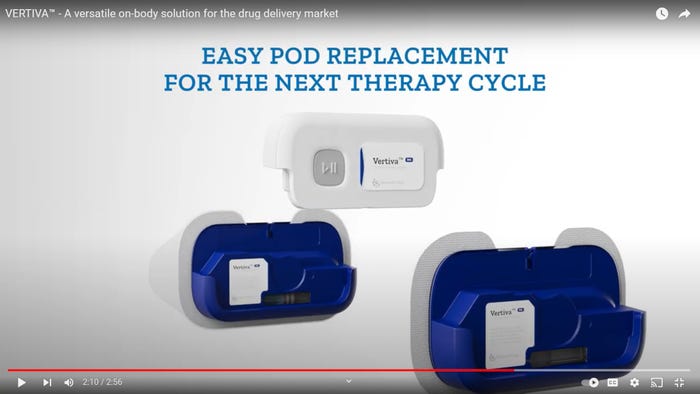
Drug-Delivery Devices
Pharmapack Europe 2024 packaging innovation examples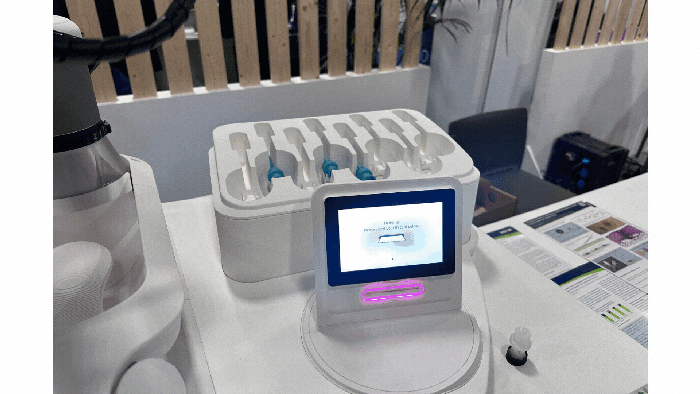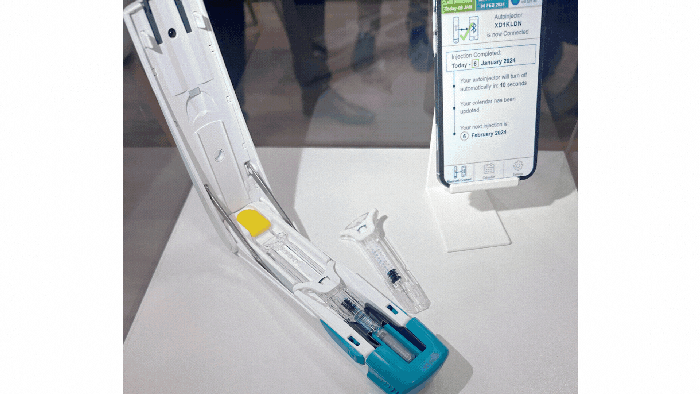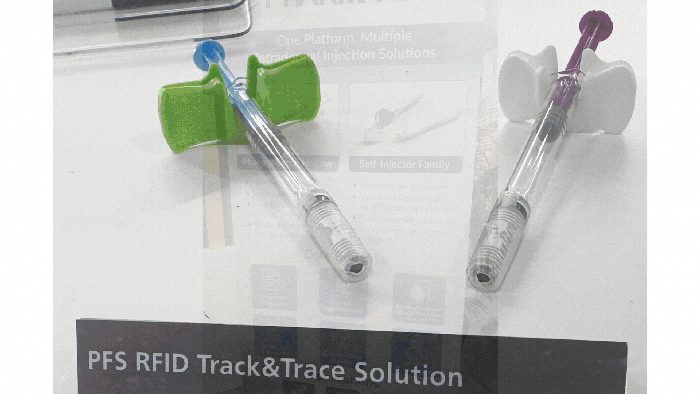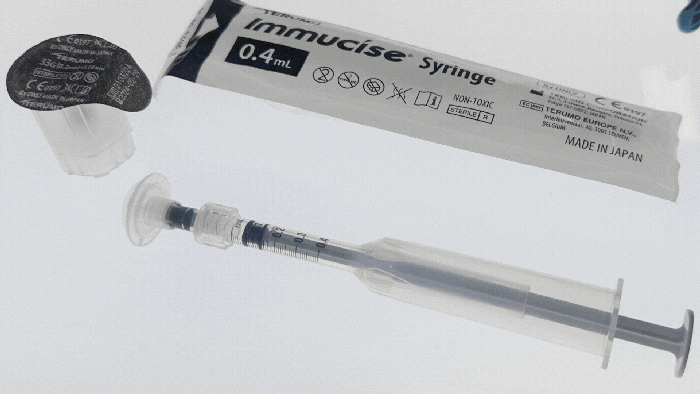
Pharma & Medical
4 Observations from Pharmapack Europe 20244 Observations from Pharmapack Europe 2024
Industry experts Mindy Katz and Mathias Romacker from the professional service organization Kymanox share their insights from what they saw and heard in Paris.
Sign up for the Packaging Digest News & Insights newsletter.