November 12, 2015
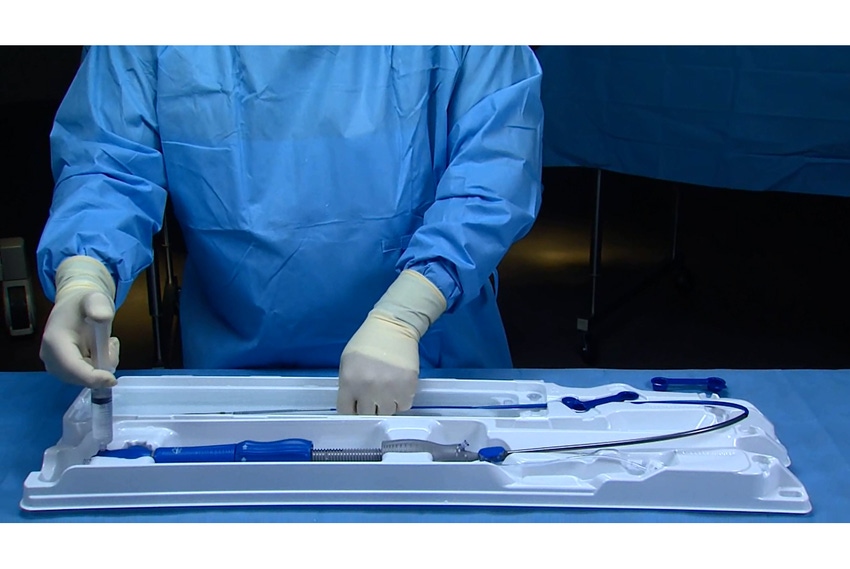
Finite elemental analysis and customer input helped guide the novel packaging developed for Medtronic’s latest generation of TAVI heart catheters that cut operating room labor by half.
The award-winning EnVeoR Transcatheter Aortic Valve Implantation (TAVI) System from the Cardiac and Vascular Group at Medtronic Inc. is a next generation breakthrough on several levels. The packaging was recognized with an Innovation Excellence in Technological Advancement and Enhanced User Experience in the DuPont Awards for Packaging for the packager and for packaging vendor Prent Corp.
Introduced in September 2014, the product has been sold into markets in Europe (where it is CE marked), Latin America, Australia, New Zealand and many other countries.
Among a number of feedback inputs from stakeholders was adding functionality to the tray housing the catheter within a sterile environment. The team also sought to reduce packaging components and waste where possible.
Application of ethnography techniques, i.e., contextual observation, were undertaken by the design team to understand the needs of the users and come up with a unique and innovative design to address these needs. In doing so the design team pushed the boundaries of the tray design and functional requirements through a customer-driven “Gemba” and “voice of customer” (VOC) process.
The result was the EnVeo R TAVI System, an improved successor to the company’s CoreValve system. It offers an innovative packaging configuration that provides a single user—two were required before—with complete control of the catheter and additional functionality during the preparation and loading of the valve onto the catheter. The 62 in.-long catheter is significantly larger in size and weight than that of other products familiar to cardiologists such as coronary balloons or stents.

The packaging that consists of a tray in a single pouch housed inside an SBS carton replaces a double-pouch configuration used previously. This improves the ease of handling in the Cathlab (Catheter Laboratory operating room). The added functionality of the tray removed unnecessary clutter and tasks from the catheter loading team and helped to streamline the valve preparation and loading process.
A new film laminate was custom developed to meet the stringent medical device packaging requirement.
Time-reducing finite elemental analysis
In order to optimize the tray design, finite element analysis (FEA) techniques were adopted to implement particular tray to ensure robustness prior to manufacture. FEA helped to reduce the packaging development cycle by 4.5 months and resulted in meeting project timelines, Medtronic reports. FEA transformed tray development from a design, test, iterate model to a predictive model where weaknesses are identified upfront and removed before prototyping.
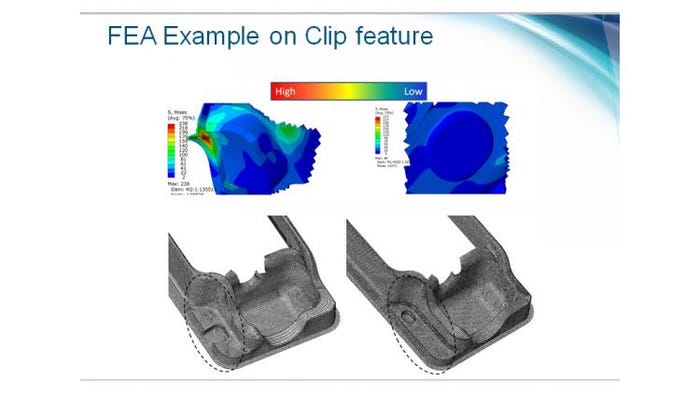
Along with a more complex design, the team selected a new tray material. This allowed the additional complexity to be added to the tray without compromising on structural integrity. The team also looked to add additional components to the tray to assist in its use in the operating room.
The innovative tray allows a single user complete control and additional functionality during the preparation and loading of the valve onto the catheter. A reflective film strip positioned within the integrated loading bath of the tray gives the surgical nurse a 360 degree visualization of the valve and all the attachment points to provide confidence and comfort of precise valve loading on the catheter.
A unique swivel feature in the tray design, along with locking features, allows the tray to remain in a straight configuration during transportation before it is manipulated using the swivel into a U-shaped configuration during valve loading just prior to surgery.
With the new tray, aspects of the loading process are streamlined to reduce the burden on the operator, while still maintaining the tray’s primary role to contain the catheter in a sterile environment and deliver it securely to the end user. It is claimed that the tray helps reduce the labor needed for catheter preparation at hospitals by 50%.
All these changes represented significant technical challenges in meeting the strict biocompatibility, sterility and functional testing requirements of the packaging configuration.
-edited by Rick Lingle, Technical Editor
You May Also Like


