September 26, 2016
Galafold was recently approved in Europe as a first-line, long-term monotherapy for Fabry disease in patients with amenable mutations. The Almac Group has helped Amicus Therapeutics Inc. with Galafold’s packaging along the way, first working with the drug company in 2009 when it needed an outsourcing partner for Phase III clinical manufacturing and packaging of its solid oral AT1001 compound. Almac’s Pharmaceutical Development and Clinical teams have continued to work with Amicus to advance the drug product through scale up, registration, and now into commercial supply.
“Providing quality drug product and sufficient drug supply is critical to ensuring a successful launch of Galafold for Fabry patients who have an amenable mutation,” said Enrique Dilone, Ph.D., RAC, Senior Vice President, Technical Operations at Amicus Therapeutics Inc., in a statement. “Almac has been an outstanding outsourcing partner for Amicus, and we look forward to continuing the relationship as Galafold becomes available across the EU.”
To support Galafold’s launch across Europe, Almac manufactures and packs Galafold at its United Kingdom commercial facility in Craigavon, Northern Ireland.
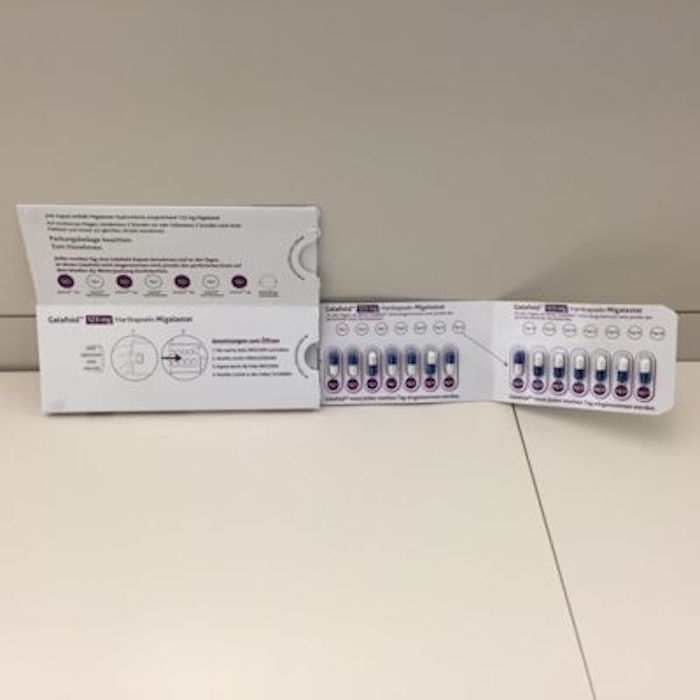
Considered an orphan drug, Galafold is supplied as an immediate-release hard capsule in PVC-PCTFE-PVC/Al blisters contained in a wallet/Dosepak presentation, explains Stuart Hunter, Packaging Design Manager, Almac Group. “With a dosing regimen of one capsule every other day, patient compliance was a key requirement when the final pack format was being designed. Child resistance and senior friendly packaging was also required.”

Hunter tells PMP News that the blister/wallet design used throughout the clinical studies evolved from the simple blister/wallet pack to what now is the approved commercial package, a child-resistant, senior-friendly format that aids patient compliance.
“Throughout the clinical trial process, Amicus engaged with patient focus groups to gather their valuable feedback on the packaging format,” says Hunter. “This, together with input from the Almac multi-disciplinary project team (including Packaging design, production, and engineering) and the specialist packaging personnel at the [Dosepak] component supplier Westrock, the final pack format was agreed.”
The team’s challenge: design the package in a way that would encourage patient compliance with an “every other day” dosing regimen. Amicus together with Almac’s in-house packaging team designed a compact monthly wallet pack. The design “guides the patient through the dosing regimen with innovative design aspects such as an area to note start date, a clear day 1 to 28 schedule and ‘non dosing days’ being identified with perforated circles that would then be punched out,” says Hunter.
Currently only one SKU for Galafold is marketed, with plans for further country launches. “One pack presentation will service all European markets, but with each European market having its native language and associated printed artwork,” says Hunter. “Minimizing stock holding for smaller markets, Amicus has launched a Nordic pack--this is its first multi-country / multi-language pack. Implementation of further regionalised packs may prove useful to Amicus as the launch of Galafold is rolled out to more EU countries.” Amicus is currently submitting regulatory applications in other markets, and it is envisioned that Almac will service those markets from its Craigavon facilities, he adds.
The wallet features built-in tamper-evident solutions as well as the capacity for serialized data to be applied as required.

“It’s great to see the launch of this precision medicine addressing a debilitating unmet medical need, and it is gratifying to have played our part in its development and commercialization,” states David Downey, Vice President, Commercial Operations at Almac. “Amicus drew upon many of the services Almac has to offer, from development, through clinic, and into commercialisation. We look forward to a growing partnership as Amicus services the needs of the Fabry patient population who have an amenable mutation.”
Almac expanding in the United States
The Almac Group has just announced a $5.2 million investment and the creation of almost 80 new jobs across its Durham, NC, facilities. The company is currently celebrating 20 years in North America: In 1996, Almac opened its clinical trial operations in Audubon, PA; by 2000, it had expanded its North American presence into North Carolina through the acquisition of Applied Clinical Concepts Inc (ACCI) and Duke Clinical Research Institute Pharmacy (DCRIP), which merged to form Clinical Trial Services based in the Research Triangle Park, Durham.
“This latest investment and increase in capacity at our Durham facility is a sign of our continuing commitment to offer market driven solutions to our client base,” said Donna Christopher, Global VP Operations, Almac Clinical Services, in a statement. “We are delighted to mark our 20th year in the US with such a significant announcement – once again reinforcing our dedication to global expansion.”
The $5.2 million investment will support the expansion of its Clinical Services facility along with the development of its Diagnostics and Clinical Technologies operations based in the same area. Almac currently employs more than 1600 individuals across 6 facilities in the United States and almost 3000 people based in Europe and Asia.
Commenting on Almac’s 20-year milestone in the United States, Robert Dunlop, President & Managing Director, Almac Clinical Services said: “Almac’s commitment to our customers remains as strong as it was when we first opened in the U.S. twenty years ago. Our ongoing success would be impossible without the support and loyalty of our dedicated employees who remain committed to Almac’s vision. We are delighted to announce further investment in our Durham facilities enabling us to continue to support our clients’ needs and meet the increased demands for our integrated services and I look forward to our continuing success over the next twenty years and beyond.”
About the Author(s)
You May Also Like




