Technology alone cannot create winning combination devices. Devices must be designed to support safe and effective use and to mitigate use errors.
April 23, 2021

The last decade has witnessed an onslaught of home-based technology, from smartphones with their integrated cameras, apps, and wireless communications to voice-activated web appliances like Amazon’s Alexa and Google’s Home providing wirelessly monitored control of nearly anything we can imagine. While this has been wonderful for consumer convenience, it is also enabling at-home patients to do much more to support their health thanks to this new digital age. Along with advancing vital signs monitoring, patients can collect their own samples for lab analysis and perform diagnostic tests and therapies, including bolus drug dosing to continuous monitoring and real-time optimized delivery with wearable devices. This sector of the industry is growing so precipitously, FDA felt a need to create a new classification of medical products known as Combination Devices.
This article highlights the benefits of rigorously integrating usability design into the development of patient or layperson-operated combination devices used in home-based healthcare.
What Is a Combination Device?
Combination devices are a recently isolated ecosystem that was intended to address shortcomings resulting from the convergence of two traditionally independent industries—pharmaceutical and medical device manufacturers. A combination device is essentially a delivery mechanism (device and/or biologic) coupled to a drug source that transfers the drug onto or into the patient’s body for diagnostic or therapeutic purposes. In the Code of Federal Regulations under21 CFR 3.2(e), FDA cites examples of combination devices as:
A product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are physically, chemically, or otherwise combined or mixed and produced as a single entity;
Two or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and biological products, or biological and drug products;
A drug, device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, device, or biological product where both are required to achieve the intended use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need to be changed, e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in dose; or
Any investigational drug, device, or biological product packaged separately that according to its proposed labeling is for use only with another individually specified investigational drug, device, or biological product where both are required to achieve the intended use, indication, or effect.
Traditional examples of patient-use combination devices include oral inhalers (dry powder or aerosolized fluid), eye/ear/nose drops, prefilled syringes for chronic drug delivery (insulin, pain management), and pen injectors (epinephrine and allergy therapy). More recently, wearable combination devices such as pain-management patches and insulin pumps are bolstering this rapidly growing market sector, requiring greater cooperation between these behemoth industries with increased regulatory scrutiny of safety and efficacy as potential use-risk skyrockets along with their benefits to the quality of healthcare. (Research from Grand View Research projects a growth rate [CAGR] for connected health and wellness devices of 19.6% from 2019 to 2024.)
Advancing Combination Device Growth
While the on-paper benefits of greater convenience and lower cost provided by distance care for routine and repeated drug delivery is clear, widespread adoption of such practices has been tepid. This slow reaction by the community is partly due to social inertia (institutional change is never rapid), lack of quality patient-use devices, and mistrust by healthcare providers that patients can actually manage these routine tasks with accuracy and reliability. However, a recent cataclysmic change has turned this corner of the healthcare paradigm on its ear—COVID-19. According to the Department of Health and Human Services, at the start of the pandemic in February 2020, a mere 0.1% of Medicare primary care visits were conducted remotely via telehealth. By April, that percentage had increased more than 400 times to nearly half of all visits (43.5%). While the virus forced the change in routine practices due to infection risk within traditional healthcare facilities, in less than a year, societal reaction has been to embrace distance care due to convenience, along with a continuing desire to manage infection risk.
It might be expected that after the world is immunized from the 2020 pandemic, there will be a desire to return to traditional practices of in-office, lab, and hospital visits. But what if the healthcare industry could wake up to a simply better way of conducting routine procedures by offering bespoke devices that empower patients to provide the same services with similar levels of quality as traditional clinical care? To accomplish this, a few things have to happen simultaneously. Along with providing better devices, reimbursement coverage must be developed to create economic incentives, and healthcare providers must be able to confirm that their patients are compliant (did they conduct the task?) and adherent (did they administer the task correctly, accurately and on schedule?). The good news is that compliance and adherence can be ascertained using secure digital authentication.

Emergence of the Digital Health Age
First, a little history on digital health. It wasn’t long ago that patient-operated combination devices did anything more than deliver routine medications via rather low-tech mechanisms (inhalers, needles, drops, and aerosols). The advent of available smartphone technology, coupled with dedicated software apps, add-on USB-connected sensors, and firmware, began what is proving to be sustainable growth in home-based therapies. By embedding real-time sensors and diagnostics into modern combination devices, intelligent algorithms can adjust dosing and control routines to administer more effective therapies based on actual patient-produced data. Without incurring much up-front cost, the layperson can easily conduct routine drug delivery, then upload data via internet or cellular connection to the professional caregiver for oversight.
At some point along the way, this digital or “mHealth” industry struck a roadblock with regulatory agencies that were rightfully concerned over quality, reliability, and accuracy issues associated with digital health products. While medical devices must be developed with a level of rigor and quality required by regulatory bodies, the same is not true of consumer devices. Because these mHealth providers were heavily leveraging the low-cost opportunity to piggyback on existing personal electronic devices (personal computers and PDAs, which were replaced by smartphones), this roadblock was a serious setback to an industry eager to develop an impressive array of devices to help improve healthcare while reducing costs. An adjunct to the problem was the real risk that these early at-home solutions could present false-negative diagnostic results, encouraging potentially deadly non-action by the patient. As a result, most caregivers preferred to continue prescribing routine in-office or hospital-based check-ups, negating the intended value of distance-care.
In the ensuing decade since these early attempts to advance the mHealth industry, smartphones have grown ten-fold in sophistication. Elaborate encryption and multiplexed connectivity to back-office super-computers featuring onboard artificial intelligence have enabled both reliable and private data transfer to create what is affectionately called “big data.” These are massive banks of globally compiled and processed, HIPAA/GDPR-compliant healthcare information. These ever-growing databases are seeking to provide superior diagnostic prognoses based on historic, DNA-tagged patient outcomes, coupled with individualized therapy guidelines. Ineffective and adverse drug interactions can be a thing of the past with customized medicine. Slow industry adoption of these new advancements was met with heavy public and political criticism over what was deemed over-conservative oversight by regulatory agencies, crippling digital health innovation. With burgeoning healthcare costs, there needed to be a paradigm shift to improve patient outcomes at lower cost and burden to an ever-shrinking clinical workforce.
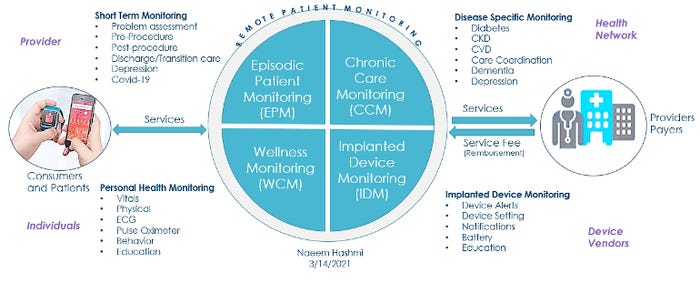
Above: Examples of solutions for remote patient monitoring. The above image by Naeem Hashmi was presented during the April 7 MD&M BIOMEDigital panel discussion, "Design Trends in Wearables and Patient-Operated Devices." Hashmi, who serves as digital health solutions strategic advisor, Boston Scientific, spoke on the panel along with author Philip Remedios; Mark Milton-Edwards, head of health solutions - digital health, Teva Pharmaceuticals; and Machiel van der Harst, founder & CEO, Tech 5. The panel discussion is available on demand through May 7. Click here to register. (Image published with permission from Naeem Hashmi.)
Regulatory Efforts to Spur Innovation
The challenges faced by the digital health industry over the last decade has propagated a few government initiatives (https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/21st-century-cures-act) to encourage innovation in this space. Addressing the need to monetize distance-care, the Centers for Medicare & Medicaid Services (CMS) launched the Medicare Physician Fee Schedule and Quality Payment Program in 2019 intended to encourage remote patient monitoring and telehealth programs via direct reimbursement. This effort followed earlier efforts by Centers for Medicare & Medicaid Service (CMS) with CPT code 99091 (https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018) to specifically reimburse clinicians for time spent collecting and interpreting transmitted patient data.
Thanks to reimbursement revisions, many healthcare providers now offer telehealth in their coverage plan offerings, no doubt catalyzed by the recent COVID-19 pandemic. These portals bring the virtual clinician into the home to troubleshoot and talk patients through challenges while also providing medical knowledge. Never before has it been more convenient to conduct routine tasks from the privacy, comfort, and safety of one’s own living room. This distance-care phenomenon is accelerating in popularity as today’s elderly patients are increasingly less tech-averse, comfortable with wireless interoperability, and express a desire to be better informed and involved with their healthcare. This emerging mindset demands a clearer understanding of the disease state, individual prognosis, and sense of control of one’s own future. The hope is to reduce overall healthcare costs via self-care opportunities and early diagnosis of serious emerging illnesses through smarter and continuous monitoring of high-risk symptoms.
It is this essential link to a viable economic model that will fuel available technology that can rapidly advance quality and meaningful distance care. With full support from clinicians and healthcare providers, combination device manufacturers can have the missing ingredients to evolve the healthcare model from reactive to preventative, or at least with continuous wearables therapy, slow down the advancement of chronic illness, which today amounts to 85% of total healthcare costs in the USA.
User-Centered Design an Essential Element in Patient-Use Combination Devices
Unfortunately, digital healthcare technology alone cannot create winning combination devices for patients and the healthcare industry. While clinicians are rigorously trained to precisely follow protocols in a (relatively) clean, well-controlled environment, patients operating out of their home or on the road have no such routines or controls in place. Consider the limitations of self-actuation (single-handed operation, reach and sightline limitations, pain-induced motion during critical steps) of a combination device by a relatively unskilled end-user, operating in an unpredictable fashion within an uncontrolled environment. These are very real design requirements for a “distance-care” device that simply do not apply to clinician-operated predecessors.
In order to develop devices that meet safety and efficacy standards, medical device designers must mitigate use-errors associated with the most notorious patients. Bad habits like smoking and other substance abuse, unpredictable discipline, non-compliance/adherence, and hygiene habits coupled with personal comorbidities like cognitive impairment, memory, eyesight, strength, and dexterity loss add to the challenges in providing reliable and repeatable solutions to such an end-user.
Conducting early and thorough usability studies that characterize intended end-user personas prior to initiating the design process is fundamental in identifying critical requirements that will produce devices that overcome these unavoidable use-related challenges. By identifying these specific use-cases before the design begins, the team can then produce potential solutions that can be legitimately validated against those requirements. This user-centered design process is so fundamental in the creation of critical medical devices, the FDA now requires documented evidence that the process has been followed and that formal user testing indicates the resulting design is safe to use. When the user is the patient, the process becomes even more fundamentally critical. Therefore, these patient-operated products can no longer be retroactive adaptations of clinical devices originally intended for highly skilled and trained clinicians. They must be designed from the ground up for patient operation in the expected distance-care environment.
User-centered innovation, coupled with continuous monitoring, has enabled even sensitive drug-delivery (wearable insulin pumps) and other critical care systems to be performed by the patient. Remarkably accurate and self-calibrating “medical-grade” sensors guide largely automated devices to do most of the work for the patient, much like autonomous driving aids in modern automobiles. The future smart operational architecture mitigates use-risk while providing real-time trending data uploads to the caregiver for further interventional consideration. The user experience (UX) is optimized by including consumer product-like touchscreens with full gesture control, voice-to-text, and commands. Remote and automatic intervention may even assist the smart distance-care combination device in adverse and emergency situations (calling EMS, resetting therapy limits to safe levels, reversing a procedure).
Then there is the subject of patient-as-consumer. Nobody likes to broadcast to the public that they have health issues, and this mindset pertains most obviously to wearable devices that must travel with the patient in the public domain, but it still holds true within one’s home as well. When they cannot be hidden from sight, these devices must be aesthetically pleasing and consumer-like in appearance to conceal their actual function while still remaining rugged, easy to use, and easy to maintain. User notifications require subtlety such as a silent/vibration mode, pleasant sounds, and visual alerts while still remaining effective. If all of these consumer-centric attributes can be offered by future designs, we can expect the patient to confidently and enthusiastically assume control of many activities that elevate the quality of their own healthcare. In so doing, we are freeing up tomorrow’s overworked clinicians to intervene only when human judgment and decision-making trumps artificial intelligence.
Biometrics and Authentication
Beyond operational performance, and in order to elevate the level of care of these remote-use devices, punctuality and accuracy of operation (adherence) is essential to support the elevated sophistication that technology can provide. Device companies must be able to verify whether the user is following protocol or falsifying input data, exchanging samples with others, or modifying collection or procedural parameters such as before or after meals, time-of-day, before or after exercise, etc. The latest biometric authentication technologies use finger, palm, retina, and facial scanning to create a HIPAA/GDPR-compliant digital signature or identity. Thereafter the patient can be digitally identified via real-time video tracking of the controlled procedure while also complying with privacy laws. This authentication process can therefore detect “off-label” use and appropriate intervention while also disqualifying falsified data. Ultimately the system is intended to encourage strict protocol compliance, verifying to the caregiver and the insurance provider that these remote procedures are carried out accurately and that data from compliant patients can be relied upon to inform the therapeutic optimization at every interval.
While it is a process, the medical device community can gain trust and confidence with the distance-care model by extending reimbursement coverage to provide economic incentives, thereby encouraging device companies to continue investing in the development of sophisticated patient-use combination products. The best-of-the-best combination devices are intelligent, connected, and well-designed wearable devices that deliver continuous monitoring and drug delivery comfortably and inconspicuously, eliminating cyclical shock to the body that traditional bolus-based systems simply cannot avoid. Continuous therapy is safer and more effective for patients with chronic conditions, slowing their advancement, severity, and improving one’s quality of life. By improving simplicity, accuracy, capability, and safety, even comorbid patients are encouraged to comply with and advance the level of their own care and education. All sides win when early intervention can prevent, reduce, or manage catastrophic illness, at lower cost and without large scale interruptions to one’s lifestyle. Avoiding unnecessary exposure to infection is the icing on the cake in a post-COVID-19 world.
About the Author(s)
You May Also Like




