December 11, 2015
Donald S. Barcan
Packaging process validation is a given. Not only has FDA issued a guidance document about process validation, but many packaging departments at medical device companies have recognized the practicality and productivity of monitoring and controlling packaging processes.
One of the most efficient processes for packaging medical devices is the use of a form-fill-seal machine. Because the machine has so many mechanisms working in conjunction to form, fill, and seal two webs of materials, it is imperative that engineers carefully monitor each process parameter to ensure the quality production of sterile medical device packaging.
Makers of form-fill-seal machinery have made monitoring a form-fill-seal process for validation purposes much easier by adding certain tools to new machines. However, because purchasing a new form-fill-seal machine requires a significant outlay of capital, many device manufacturers cannot take advantage of the new methods for monitoring critical machine parameters offered with new machinery. Alternatively they must retrofit their systems or use stand-alone units to monitor parameters and keep them in check.
One solution is adding a data acquisition system to existing machinery. This article will address the risks inherent in form-fill-seal processes and how to monitor many of them using a retrofitted data acquisition system. By monitoring the data acquired by the system, it is much easier to validate and control the process.
Why and What to Validate
Most guidance documents discuss the objective of process validation as "the documented procedure and process for obtaining, recording, and interpreting the results required to establish that a process will consistently yield product complying with predetermined specifications." In the case of packaging equipment, the objective is to first define and understand the critical parameters of the process that affect the equipment's ability to meet the package specifications.
The critical parameters listed here and in Table I include both parameters that can be measured in real time and those that cannot.
The real-time measurement critical parameters are forming temperature(s); forming pressures; forming timing; forming chiller differential temperature; sealing temperature; sealing temperature distribution; sealing pressure; sealing dwell time; sealer chiller differential temperature; input voltage; heater current; room temperature; and date and time.
And the non–real-time critical parameters are quality of tooling; forming film minimum thickness; forming registration; product loading tolerances; sealing registration; sealing pressure uniformity and distribution; sealing gaskets; seal strength and seal strength uniformity; print registration; and cutting registration.
There can be, and usually are, additional processes that would also need to be included in the process validation analysis. Examples involve the in-line printing of the top web and equipment for automated filling or product loading. Even though these are both important issues (improper print copy still accounts for the major number of defects leading to package adulteration), they will not be included in this discussion.
Causes and Effects of Equipment Problems
Table I lists the major parameters and their risk to package integrity. Though geared to form-fill-seal equipment, the table is applicable to other processes such as tray and pouch sealers. For tray sealers, the critical process parameters are sealing temperature, sealing temperature distribution, sealing pressure, and sealing dwell time. All of these parameters affect the packaging process.
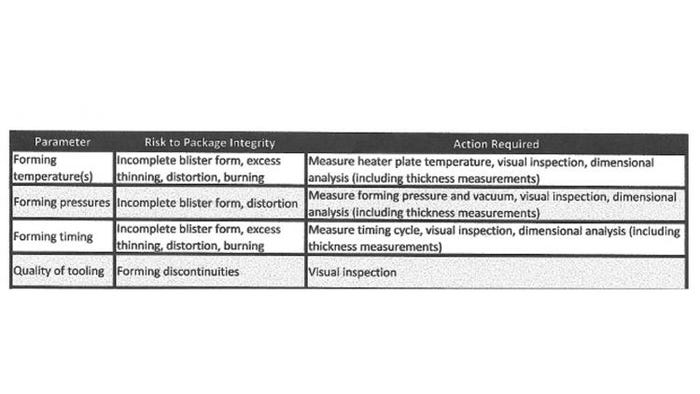
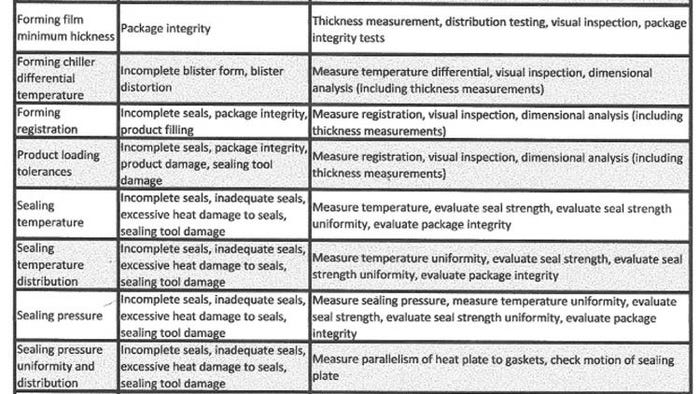
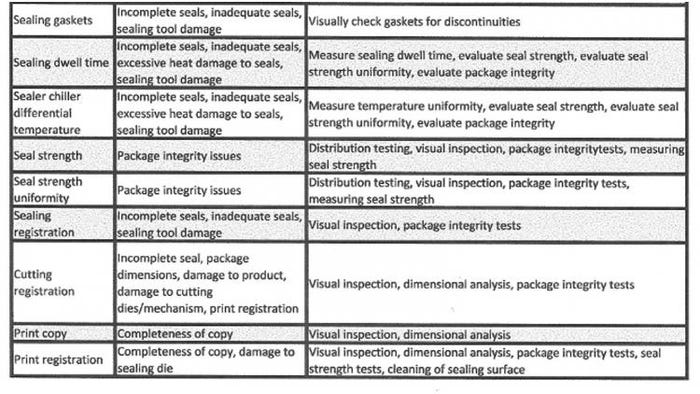
Table I. Major parameters of a form-fill-seal process, their risk to package integrity, and the action that must be taken.
How to Validate and Monitor
Much of the form-fill-seal packaging equipment currently in operation does not have precise sensors for evaluating the critical parameters just described. In most cases, the instruments controlling the process are the same as those displayed. In addition, these vital and key parameters are not being electronically recorded. The recording of these parameters is an essential ingredient in the device history record, which can be used in cases of process control inquiries.
Unfortunately, using the same measurement parameters that control the process to monitor process uniformity is not the desired method for monitoring process control. Most experts agree that process monitoring should be conducted using independent process indicators.
Several manufacturers currently, or will shortly, offer validation or data-acquisition packages as accessories for their equipment. However, these "packages" are for new equipment. Manufacturers whose existing equipment lacks such accessories must look for retrofit packages or buy new equipment.
Vinatoru Enterprises Inc. (VEI; Pompton Plaines, NJ) has developed a commercially affordable system called Data Acquisition System (DAS). The unit is currently in use on several form-fill-seal machines and costs in the range of $15,000 to $25,000.
This add-on DAS monitors and records the designated processing parameters. The system's presettable alarms advise the machine operator if the process deviates from the set points and is no longer under control. DAS includes all hardware, including NIST-calibrated sensors and extension cables, a PC supplied by Dell Computer Corp., ISO 9002–compliant data-acquisition cards from National Instruments, and proprietary and validated software that is compatible with Microsoft Excel and statistical software.
Installation applications vary, so DAS was designed to be flexible enough to adapt to most needs. The current system can monitor up to 14 process parameters. However, it is possible to monitor as many parameters as required. All sensors are accessible for periodic calibrations.
Examples
Having control over sealing temperature is key to producing well-sealed packages. Using DAS enables the user to monitor more than one temperature location at a time.
Suppose that a user has a complicated sealing die involving the sealing of 12 cavities. It would be inadequate to assume that monitoring one spot of the sealing die is representative of the total sealing performance. For example, if a cartridge heater near the outside of the tool burns out, the immediate impact will probably be a lower surface-sealing temperature for the packages at the outside of the tool. Improper sealing of one or more of the 12 cavities, which a single point sensor might not detect right away, could result.
Let's consider another example. In a form-fill-seal process, the use of chilled water is necessary to keep the forming and sealing stations cool enough to prevent machine operators from burning their skin. Though the chilled water is effective, the chilled areas form a thermodynamic balance with the needs for forming and sealing. The DAS would monitor the input and output water temperatures and sound an alarm if these temperatures were outside the predetermined limits.
Conclusion
Providing effective process control is no longer a luxury. For medical devices, it is a necessity, as mandated in the current FDA regulations. Now the tools to ensure process control are available to packagers no matter what the age of their equipment.
This article was written by Donald S. Barcan, president, DBI (Donbar Industries Inc.) and Mihai A. Vinatoru, president, Vinatoru Enterprises Inc.
You May Also Like


