December 8, 2015
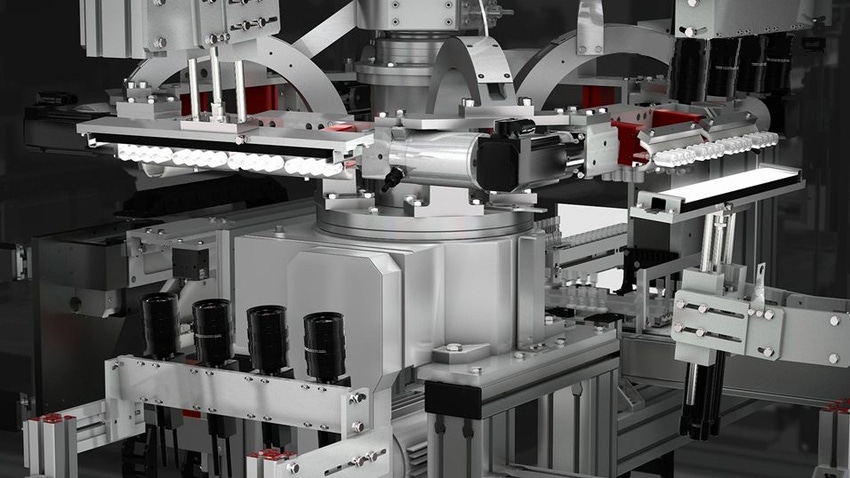
Blow-fill-seal packaging offers an alternative for companies interested in replacing glass containers or IV bags for both small-volume and large-volume parenteral drugs. Given the aseptic nature of the process and the higher automation levels, blow-fill-seal packaging results in fewer particles, reports Tim Kram, general manager for rommelag USA Inc.
All processes present some risk of particulates, Kram explains. But “blow-fill-seal is a 3- to 12-second process that inherently has fewer particles from outside the process, such as were found in the product recalls this year,” he says.
“And compared with glass vials, which involve manufacturing at one site, transporting them, then washing and depyrogenation, and finally having them sit open exposing them to particle contamination, BFS containers have a lower risk,” he says.
Kram adds that “the luer-type BFS containers are designed to be opened and used with a traditional syringe. Similar to glass ampules, without the risks associated with breaking open glass.”
The technology is getting another boost with rommelag’s development of automated visual inspection systems for particles. Automatic inspection systems have been developed for glass vials that “spin and stop” containers to look for particles, Kram notes. Rommelag’s new system uses vibration on a stationary block of containers to agitate a particle so it can be detected by the camera system.
The particle-detecting systems are designed for 100% inline inspection at typical BFS line speeds, Kram says. “The systems vibrate the BFS containers and then take pictures of anything that moves. We can take a picture from the bottom to detect anything heavier than the drug product, and then from the top to detect any floating particles,” he explains.
BFS containers have traditionally been visually inspected by people. “The standard approach is for a human operator to hold a container in front of a white screen for five seconds, then in front of a dark screen,” he says. “The visual particle detection standard is set by particles that are found 70% of the time from the test set.”
During validation a risk assessment is performed to determine the set of possible particles that could be present in the container. “Through risk analysis, you have to determine what types of particles for which your process is at risk,” Kram says. When using a time pressure dosing system this includes four internal sources: “metal particles from the filling mandrels, gasket materials, sealing materials, and plastic from the container itself,” he adds.
Inspection is dependent on the container design and the solution being inspected. Due to the clarity of the BFS plastic container, the threshold for particle size detection is larger than found in glass containers, he explains. This risk is lessened owing to the low intrinsic and extrinsic particles inherent to the BFS process. Solutions that are foaming, suspensions, or emulsions can also add to the challenge to inspect the container, he adds. “With these products, the inspection sensitivity will be lower in comparison to clear solutions,” he says. “In the end the automated system can easily outperform human inspection in most cases. The regulatory standard is to do better than humans can do, and the system meets and exceeds that standard.”
About the Author(s)
You May Also Like




