A new ultra-high-barrier film protects sensitive COVID-19 diagnostic devices – without aluminum foil – so devices can be calibrated with an RFID scan prior to package opening.

The pandemic has accelerated the development of diagnostic methods and devices, some of which come with demanding flexible packaging requirements. The reagents used in the microfluidic COVID-19 rapid antigen tests often require an almost total barrier to maintain the reagents’ concentration and protect analytes from ultraviolet (UV) degradation.
“Exposure to UV light can severely degrade the efficacy of the reagents,” notes Dwane Hahn, chief strategy officer at Paxxus. “You risk a false result if the chemicals are degraded, and the tests do not perform as designed.”
Other examples of diagnostic devices that use microfluidic reagents include blood glucose tests and illicit drugs tests. Typically, packaging materials containing aluminum foil at 0.001 inches or above are used to package these sensitive diagnostic kits. However, for one diagnostic device manufacturer, foil was not an option for its new COVID-19 diagnostic test cartridge. The extremely sensitive analytes used to conduct the test required the moisture and UV barrier traditionally provided by aluminum foil. The challenge was the need to use an RFID signal to scan the test device inside the package, thereby calibrating it to ensure accuracy prior to opening. This precluded the use of aluminum foil, metallization, or any material that would interfere with radio frequencies.
Pairing two technologies offers foil alternative.
In response to this challenge, Paxxus developed White Eclipse, a packaging film that pairs ClearFoil Z with a proprietary UV-blocking sealant technology to create a flexible pouch that eliminates exposure to outside light while also providing the highest level of oxygen and moisture barrier.
The moisture barrier is provided by ultra-high-barrier ClearFoil Z, an aluminum-oxide-coated polyester providing a WVTR (water vapor transmission rate) of 0.0008 grams/100 in² per day. ClearFoil Z is adhesive-laminated to a coextruded, high-opacity, dual-pigmented modified metallocene sealant film.
Because ClearFoil Z is clear, the UV barrier needed to come from the sealant layer. The diagnostic manufacturer wanted the outside of the package to appear white to the user. However, while white opaque films will provide light transmission results close to zero, some scattered UV light will still penetrate into the package. While small, this exposure to UV severely degrades the analytes in the test cartridge, impacting the efficacy of the test.
To address this, the sealant was produced using a proprietary modified metallocene blend with solid black pigment on the inside to block light and white pigment on the outside for premium and easy-to-read graphics. The multilayer construction creates a chemically inert sealant with complete resistance to light penetration and allows for strong hermetic seals on high-speed form/fill/seal equipment, ensuring that the chemical reagents in the cartridge perform exactly as intended.
Non-foil film supports RFID compatibility, sustainability.
Traceability is critical for all medical and diagnostic devices for quickly identifying and isolating problems that could potentially impact product supply. RFID scanning has become a popular inventory management system where a large quantity of products can be scanned with one pass and the pertinent information (such as part numbers and lot numbers) recorded directly into the system. The ability for tests and devices to be tracked and traced creates a more reliable experience from production to end use.
The manufacturer’s diagnostic tool takes RFID a step further: The RFID signal is used to calibrate the diagnostic tool prior to opening the packaging. By individually calibrating each unit prior to use, test sensitivity is enhanced and accuracy is ensured.
Since RFID signals are obstructed by all grades of aluminum due to the Faraday cage effect, the extreme barrier properties had to be achieved in another way, leading to the development of this UV-blocking, non-foil packaging material.
The absence of aluminum foil in the film’s construction also provides a number of sustainability advantages. White Eclipse is 42% lighter than the standard foil laminates that it replaces.
The absence of aluminum foil in the film’s construction also provides a number of sustainability advantages. White Eclipse is 42% lighter than the standard foil laminates that it replaces, thereby reducing distribution costs. Additionally, traditional aluminum foil composites are very energy intensive to manufacture. Finally, as an all-polymer solution, the product is suitable for advanced recycling options.
Premium appearance inspires confidence.
White Eclipse’s smooth white surface allows for rich, vibrant color presentation using either surface or reverse printing. The added dimensional stability from the ClearFoil Z allows the packaging to maintain a premium appearance off the press all the way to the end user.
While this application is used in a healthcare setting, more and more tests are performed at home. Because White Eclipse does not contain aluminum foil, it springs back when flexed or handled, which reduces wrinkling.
“The high performance and premium appearance provide the end user with confidence that they are receiving a quality product,” says Hahn. “Because the packaging is not easily damaged in transit, the end user feels safe using the device and confident that the results will be accurate.”
Flex-crack test results indicate that White Eclipse outperforms aluminum foil structures (see “Impact of Flexing on Barrier” chart below). The technology can easily be applied to a variety of applications in place of aluminum foil packaging, such as blow-fill-seal vials, surgical blades and scalpels, transmitter devices, catheters, injectables, and implantables.
“Cost is competitive with traditional foil solutions – even more so now taking into account the rising price of foil,” says Hahn “Plus, White Eclipse is readily available where aluminum foil can be challenging to acquire.”
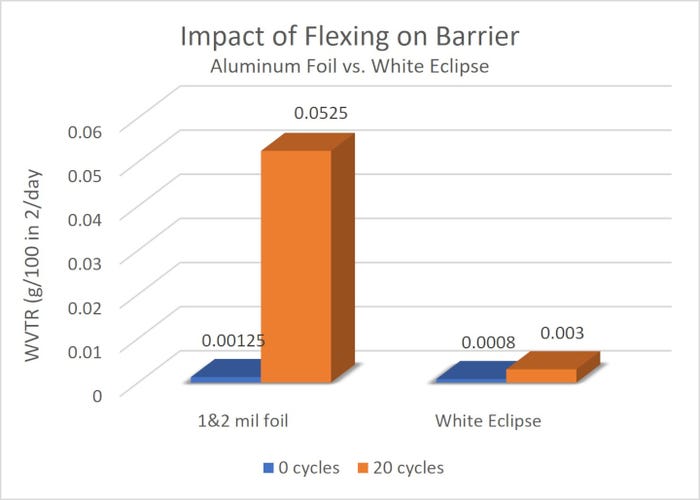
Barrier after flexing.
The chart illustrates the effect of flexing on a series of barrier composites. The only difference between the composites is the source of the barrier. All consist of 48-gauge (12µm) oriented polyester (oPET), a barrier layer, and 0.002-in. (50µm) linear-low-density polyethylene (LLDPE). Samples were flexed 10 times using a Gelbo Flex Tester and the oxygen transmission rates (02TR) evaluated before and after flexing.
Although the chart gives information on the barrier testing of regular foil versus the ClearFoil that is used in White Eclipse, the particular grade of ClearFoil used in the product is not listed.
About the Author(s)
You May Also Like




