The deadline for compliance with the European Union Medical Device Regulation is still years away, but now is the time for device packagers to begin their compliance journey. Here’s how.

Medical device makers who sell products in Europe are keenly aware of the European Union Medical Device Regulation (EU MDR) and the massive change it is imposing on their products. But they may not know how the EU MDR will affect their packaging operations.
Abhishek Gautam, director of global packaging at Morrisville, NC-based Teleflex Medical, provided an insightful look at the subject in his 2021 Virtual Engineering Days presentation: “Mitigating Risks of Massive Regulation Changes: A Practical Look at EU MDR Packaging Implications.” (The full 46-minute video of the presentation is at the end.)
Teleflex is a medical device manufacturer that operates in 40 countries and produces a range of specialty medical devices for procedures in critical care and surgery. [Editor’s disclaimer: The views, thoughts, and opinions expressed in the presentation and in this article belong to Abhishek Gautam and do not necessarily represent those of Teleflex.]
EU MDR timeline.
The EU MDR, which affects the production and distribution of medical devices, entered into force on May 26, 2017. On May 26, 2021, it applied fully; this date was originally set for 2020, but because of the COVID-19 pandemic, the European Parliament and Commission delayed the application date by one year.Additional key dates include the following:
• On May 26, 2021, the previous regulation, the EU’s Medical Device Directive (MDD), was repealed.
• Until May 25, 2024, certificates issued under the MDD before the MDR fully applied will remain valid.
• Between May 26, 2024, and May 27, 2025, MDD devices already on the market may continue to be made available.
• As of May 26, 2024, all medical devices placed on the market must be MDR-compliant.
“There’s a lot of activity going on, with medical device manufacturers scrambling to figure out how to meet the compliance,” Gautam said. “It might be easier for new products, but you think about legacy products when you think about the major medical device companies. These are big portfolios we’re talking about.”
For packaging professionals, impediments include translating MDR requirements into actionable items and balancing MDD vs. MDR compliance within the regulatory timeline. The choice between MDD recertification and re-engineering for MDR compliance may arise, for example.
Procedural compliance is important, as well. Packaging procedures, including quality systems, need to meet the intent of the EU MDR. A “consistent process to compliance,” or planning process to bring all products into compliance with the MDR, is also essential.
The state of the company’s legacy products vis-à-vis MDR compliance, or lack thereof, is another key issue to address. Retesting an existing package to establish compliance with the MDR brings up the risk of being out of compliance and needing to fill that gap posthaste.
Finally, packaging engineers must balance their regular workload, day-to-day operations, and new product introductions against the demands of ensuring MDR compliance for all of their company’s medical device packaging.
A starting point.
Packaging departments clearly need a plan for managing the complexities of a large-scale regulation change like the MDR, which emphasizes improved product handling and storage, reduced risk of contamination, better evidence of integrity, aseptic presentation of devices, and usability evaluation.
Packaging requirements under the new regulations address issues such as:
• Protecting medical device characteristics and performance during transport and storage.
• Minimizing the risk posed to patients by contaminants and residues.
• Maintaining sterility of devices throughout transport and storage, with the package’s integrity clearly evident to the final user.
• Processing, manufacturing, packaging, and sterilizing devices using appropriate, validated methods.
As packagers consider these and other EU MDR requirements, they must also keep ISO 11607 top of mind. “We, as a medical device packaging community, have to remember that ISO 11607 Part 1 and Part 2 compliance is an absolute. It’s a standard expectation. That expectation hasn’t changed as part of the EU MDR. You still have to comply ISO 11607,” Gautam stressed.
Planning for EU MDR compliance starts with a high-level, four-step process:
1. The first step is to create a knowledge base with full understanding of the EU MDR requirements and ISO 11607, Parts 1 and 2.
2. The second step is to create an essential-requirements checklist for gap assessment, based on the regulation’s requirements.
3. In the third step, the gap assessment checklist is used to review packaging designs, processes, and testing, ultimately identifying gaps in compliance.
4. Finally, in the fourth step, the team closes those gaps, strategically determining the risk of retesting a package for compliance with EU MDR vs. making changes to the package to bring it into compliance.
All four steps are important in the movement to compliance. As Gautam noted, “If you don’t plan properly, you cannot execute properly.”
Challenge or opportunity?
In his presentation, Gautam admitted that after hearing about the EU MDR for the first time, several years ago, “I was very stressed out. I looked at it as a challenge. I looked at it as a daunting task. But as I started out on that journey and created project plans, I recognized this was actually a great opportunity for medical device manufacturers to improve their products.”
He pointed out that the benefits of closing gaps in compliance, through packaging changes and redesigns, range far beyond the regulatory sphere. The manufacturer has the opportunity to make changes that update its quality systems, streamline operations, improve margins, and enhance user safety/experience, for example.
Despite the potential benefits, some medical device manufacturers might question the wisdom of making changes to packaging at this time, with the final deadline three years away. Particularly for legacy products, they may be inclined to send existing packaging to the lab to be tested for EU MDR compliance, using retesting as a way to save time and money.

But the downside of retesting is significant. “Failing a packaging test on a commercial product can potentially result in product recalls,” Gautam said.
“There’s a lot at stake,” he added, noting that a recall can lead to lost market share, stigmatization in the marketplace, and reduced stock value. “The consideration that needs to be made is whether to take a step back, and plan things in a way that you can create benefit through the EU MDR,” or take a possibly hazardous shortcut with retesting.
Keep it simple.
Considering the risks and complexities involved, how can a medical device maker simplify its approach to EU MDR compliance? For this, Gautam outlined a three-step analysis in which packaging engineers (1) categorize products, (2) recognize risks, and (3) organize and strategize.
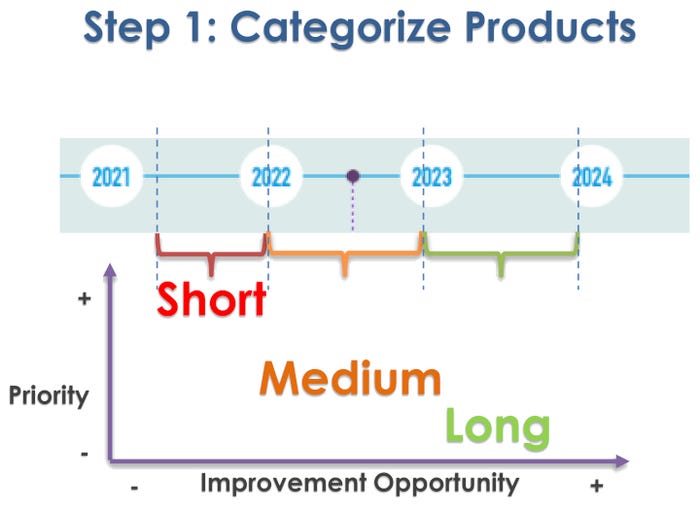
For step 1, categorizing products, it’s helpful to chart each product’s improvement opportunity over the short, medium, and long term against its priority level. Products being introduced this year have the highest priority and lowest improvement opportunity. In contrast, products due to launch in 2024 have the lowest priority but the highest improvement opportunity.
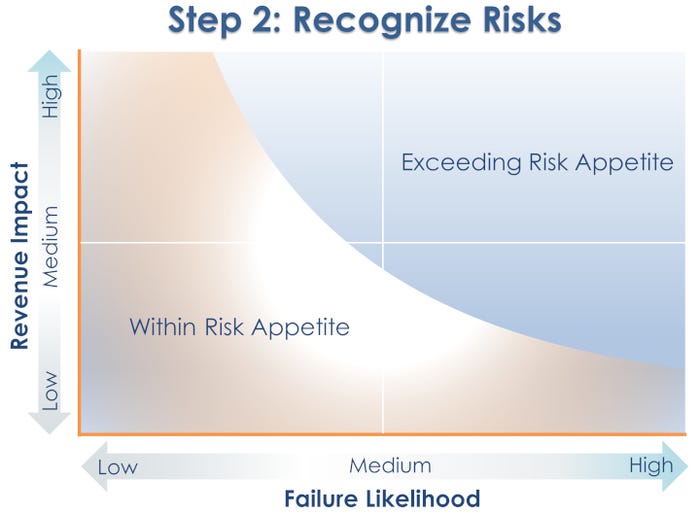
Risk recognition, step 2, uses a graph that plots the product’s revenue impact against the likelihood of packaging failure (based on simply retesting the packaging that is currently in the market). From this analysis, four risk categories emerge, ranging from low revenue impact/low failure likelihood to high revenue impact/high failure likelihood.
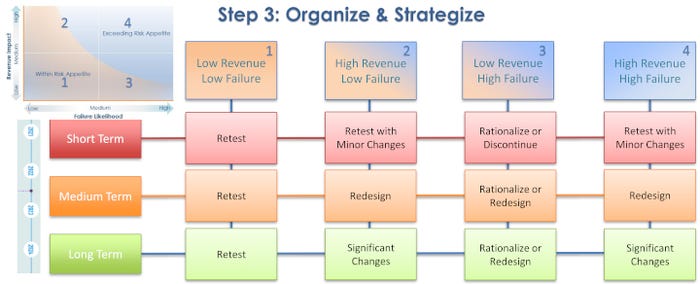
In step 3, organizing and strategizing, the packaging team evaluates each of those four risk categories for likelihood of failure in the short term (2021), medium term (2022-2023), and long term (2024). This leads to options that include retesting the package, retesting with minor changes, redesigning some aspects of the package, making significant changes, or discontinuing the product.
For products in the low revenue impact/low failure likelihood category, retesting becomes the clear choice for the short, medium, and long term. This type of product brings in very little money and has “low chance of failure. If I retest it, the chance is very limited that it’s going to fail and, by the way, it’s low revenue. I don’t have the money to spend on this, and I can’t justify changing anything about it, so I’m going to retest. These are the simpler ones to go after,” Gautam explained.
At the opposite end of the chart are products that are high revenue and have a high chance of failure during retesting. In the short term, these should be retested with minor changes; note that making changes to the package will save the product from being recalled should it fail the test.
In the medium term, packaging for these high-revenue products should be redesigned, taking advantage of opportunities to cut costs, streamline operations, and/or improve the product’s user experience and safety. And in the long term, these packages are excellent candidates for significant changes.
“Looking at the overall process, you can take all those complexities and break them down into a short-term, medium-term, long-term vision for your product filing,” Gautam said. “At the next level, you can look at the risk — revenue vs. failure — and then use this approach for organizing and strategizing. Now you are chunking up the risk in different segments and different buckets, and you are creating a plan that can help you create opportunity.”
Opportunity through redesign.
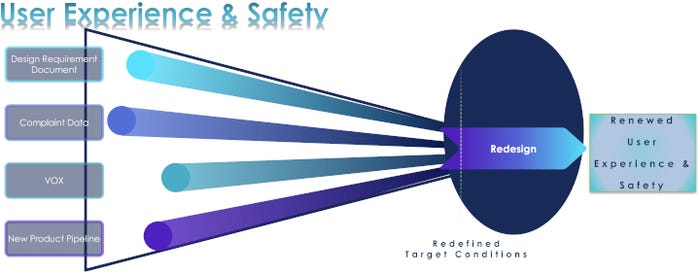
In those medium- and long-term scenarios that lead to redesigning a package, designers and engineers have the opportunity to enhance user experience and safety. Gautam described a four-pronged approach to achieving this benefit:
• Review relevant design requirement documents to get back to basics on the product and package.
• Examine complaints to understand what users are experiencing from the current package design.
• Talk to internal customers, including sales, marketing, and customer service personnel, and also to external customers to get their take on how well the package is performing.
• Look at your new product pipeline for information that may redefine the target conditions for product use and then translate that input into package design specifications.
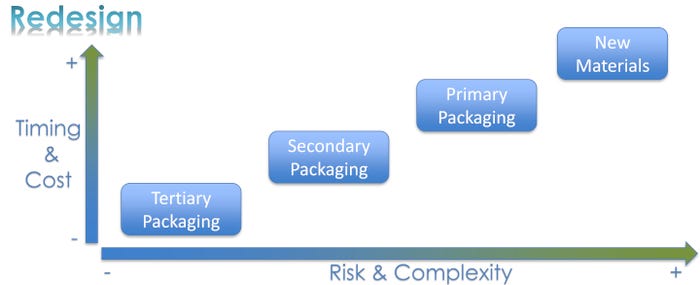
Redesigning packaging to comply with the EU MDR also offers the opportunity to reduce packaging costs. Balancing the risk and complexity of a redesign against the time and cost of the work is essential.
Moving from the lowest to highest level of both time/cost and risk/complexity, the packaging team should tackle tertiary packaging, secondary packaging, primary packaging, and new materials, respectively.
Redesigning tertiary packaging is generally simple, low-risk, quick, and cost-effective — for example, reducing a box’s dimensions or head space. At the other end of the spectrum, upgrading to an advanced packaging material may be a complex, risky, costly, time-consuming process.
Operational improvement is another opportunity inherent in packaging redesign. The same type of time/cost vs. risk/complexity analysis can be used to reap operational rewards.
Streamlining operations falls in the category of lowest risk/complexity and also lowest time/cost requirements. “Depending on your appetite for risk, you can simply streamline your operations,” Gautam said. “Maybe you want to change the assembly layout. Maybe you want to squeeze out time from your operations. Maybe you want to change the way things are organized, how the product flows, how the raw material flows.”
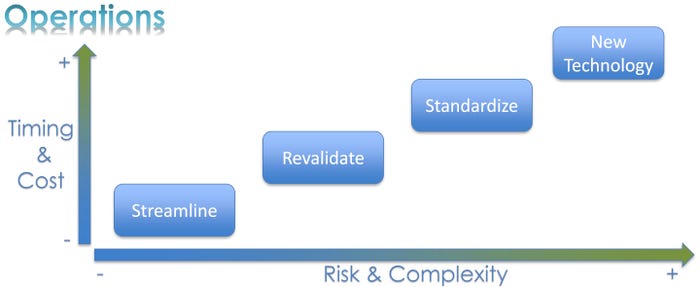
At the next highest level of risk/complexity and time/cost, a reasonable operations task is revalidation. The packaging team can review its historical process development and design of experiments and, based on that, change the parameters used to validate packaging.
The next step up, which is riskier and more time/cost expensive, is standardization within operations. Examples include standardizing packaging within or between product lines, standardizing test methods, and standardizing packaging equipment.
The highest risk level for operations is new technology. “Maybe today you are batch processing a product, and now you can go to a single-piece flow,” Gautam explained. “You can bring in a brand new piece of equipment instead of a new line, and you have state-of-the-art technology and extremely good cost on it. You’ve got great margins. The sky is the limit. How ambitious are you? What does your company allow you to do?”
He added, “From a priority standpoint, you can take those products and throw them into one of your risk buckets and say okay, this product is a great candidate for that activity. And that becomes your operations opportunity in EU MDR compliance.”
Final thoughts.
Ultimately, upgrading regulatory compliance presents medical device packaging departments with an opportunity. To take advantage of the EU MDR opportunity, the packaging team should determine what resources, internal and/or external, are required to establish compliance.
It’s also essential to have a single point of accountability for the EU MDR packaging compliance program and exceptional project management, with attention to timelines and risk management. The latter should include regular risk reviews to help the team and its internal customers get ahead of any issues with legacy products.
Finally, “ISO 11607 compliance is paramount,” Gautam emphasized. “You cannot bypass that. You cannot do things differently. EU MDR is part of it, and you have to consider that.”
About the Author(s)
You May Also Like




