
Companies that sell medical devices face far more stringent packaging requirements than the average consumer packaged goods (CPG) brand owner. To meet the needs of medical device manufacturers, family-owned Dordan Manufacturing Inc. has expanded its thermoforming plant in Woodstock, IL, to include a fully enclosed ISO Class 8 cleanroom.
Packaging Digest senior technical editor Rick Lingle toured the Woodstock facility and wrote about the new Dordan cleanroom in a recent article for our sister publication PlasticsToday. The following details about the initiative are excerpted from his PlasticsToday article.
Dordan, which is ISO 9001:2015 certified, initiated the cleanroom project to enhance its existing expertise in medical packaging. The company also designs, manufactures and distributes custom thermoformed packaging in the retail, electronics and automotive markets.
“We already have healthcare and non-cleanroom medical customers, so we are familiar with the design and quality needs of the industry,” explains Dordan marketing manager Chandler Slavin. [Disclosure: Chandler Slavin is a contributing writer to Packaging Digest.] “By constructing an onsite cleanroom, fully committing to the medical market is a natural progression for us. We have opportunities for cleanroom packaging with these existing customers and are excited to further support their packaging needs for sterile medical devices and kits.”
Dordan general manager Aric Slavin (Chandler’s brother) adds, “This new capability requires a significant investment in equipment, facility, training and talent, as well as the ancillary equipment necessary for validating the process and the part.”
The cleanroom is 50x63-ft, a 3,200-square-foot isolated room with hard-wall construction rather than “soft-style” plastic curtains. A cascading air flow in the cleanroom pushes air from the inside out. Air pressure is higher in the production area than in the gowning and material-handling spaces, where air pressure is higher than in the factory.
An air handler on the outside of the building brings in air, which is cleaned using highly efficient privacy preserving authentication (HEPPA) filters before being introduced into the cleanroom. The air circulates through the cleanroom and exits via the air return handling system.

Medical device packages produced in the new cleanroom go through critical testing and inspection.
The cleanroom, which required 18 months to build, was designed for manufacturing packaging for Food and Drug Administration Class I, II and III medical devices. During construction, the company concurrently developed new quality-control procedures and hired additional personnel.
Installed on the main factory floor in Woodstock are 11 Lyle thermoforming lines from Brown Machine. Several of these lines feature robotic devices that use suction cups to automatically remove the formed trays from the web; on other lines, trays are manually separated from the web.

(Left to right): Aric Slavin and Chandler Slavin welcomed senior technical editor Rick Lingle into the company's new cleanroom to see the thermoformer in operation.
But the thermoformer in the new cleanroom is a Kiefel Model 78.1 Premium Speedformer with Siemens controls. “We wanted a thermoformer that has a large market presence in the medical space,” says Aric, “and Kiefel is known as a medical thermoformer. Their machines are designed with the high precision and quality that’s needed.” The programmable logic controlled (PLC) Speedformer is more automated than Dordan’s other thermoforming equipment.
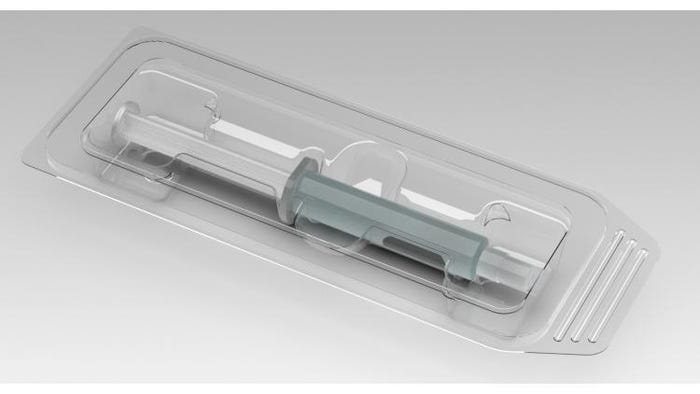
Dordan also helps with medical device package design and development.
“We are currently working with a handful of medical device manufacturers on the design and development of packaging that ensures the safety and effectiveness of their devices,” Chandler says. “These include startups, established companies bringing new products to market, existing customers that require cleanroom production and large medical players looking for new cost-competitive suppliers.”
Looking toward the future, Dordan made sure the size and layout of its cleanroom can accommodate the installation of a second thermoformer. “Many of our customers already operate in the healthcare space,” Chandler says, “and they are pleased that we are enhancing our offerings with an onsite cleanroom. We are expanding business with these customers, which has thus far been an easy transition.”
____________________________________________________________________________________________
MinnPack 2019 (Oct. 23-24; Minneapolis) is where serious packaging professionals find technologies, education and connections needed to thrive in today’s advanced manufacturing community. See solutions in labeling, food packaging, package design and beyond. Attend free expert-led sessions at multiple theaters around the expo.
About the Author(s)
You May Also Like




