Case studies in pharmaceutical and medical device manufacturing predict better unplanned downtime, improve productivity, and more.
February 2, 2021
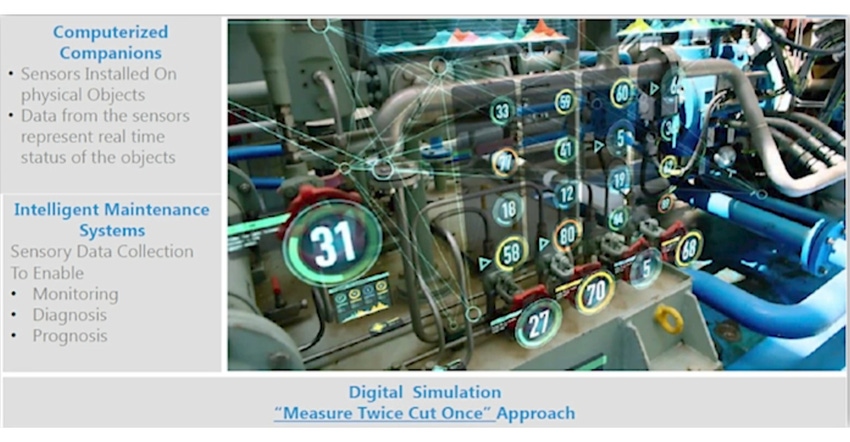
It’s one thing for a pharmaceutical or medical device manufacturer to invested in smart factory assets, and another to leveraging those assets using big data analytics. Two leaders from Birlasoft, an IT solution and services provider, provide insight and advice: John Danise, industry director for life sciences and Adavit Waghmare, vice president of digital transformation.
In their presentation “The Smart Factory: You Have All the Data, What Do You Do with It?” from 2020 Virtual Engineering Week, Danise and Waghmare begin with an overview of shifting priorities in the wake of COVID-19 and continue with perspective on new analytics solutions that eclipse the utility of prior-generation business intelligence (BI) systems. (See video below.)
The presentation illustrates such concepts using case studies. These include how a medical device manufacturer improved productivity by using sensors to connect movable equipment (tanks and packaging equipment) to the plant network, ensuring all equipment was easily located, cleaned, and sterilized. In another case study, real-time sensor data flowing from inverters, gearboxes, and motors to enable predictive models of fault conditions and analytics for reduced unplanned downtime, which was as high as 48% for some equipment.
About the Author(s)
You May Also Like


