Pharma & Medical
More Topics
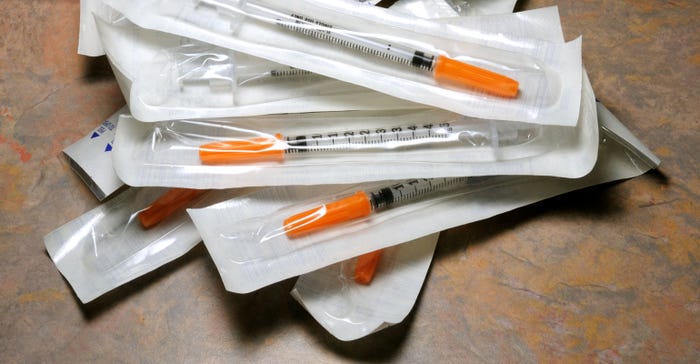
Packaging-line-Alamy-KWEGBY-ftd.jpg
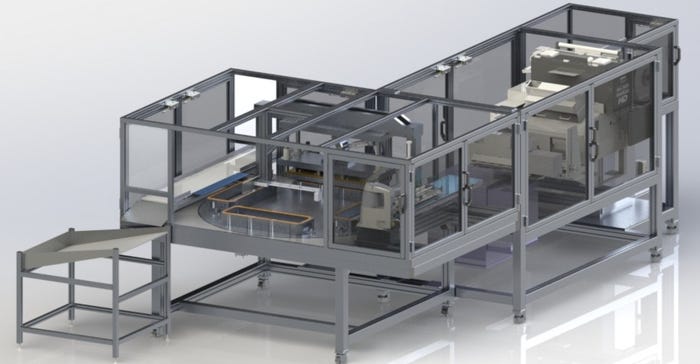
Machinery/Equipment
What’s Revolutionizing the Packaging Equipment Space?What’s Revolutionizing the Packaging Equipment Space?
Expect automated packaging equipment to gain traction as pharmaceutical and ecommerce sectors expand post-COVID-19.
Sign up for the Packaging Digest News & Insights newsletter.