January 29, 2014

Aptar Pharma Landmark dose-counting actuator
If you're among the 60 million people in the U.S. that suffer from allergic rhinitis-between 10 percent and 30 percent of all adults, and 40 percent of American children)-you know the ailment is no walk in the park. You rely on treatments and devices like inhalers to make it possible for you to step out into the world while being able to breathe, and you can't have your medication failing you by running out and catching you unaware.
To that end, the development team at Sunovion Pharmaceuticals Inc. wanted to ensure that the packaging and delivery system for Zetonna (ciclesonide)-a dry nasal aerosol intended for treatment of symptoms associated with seasonal and perennial allergic rhinitis in patients 12 and older-adequately served patients by being easy to use and reliable. The Sunovion Pharmaceuticals team contacted the staff of Aptar Pharma, a company specializing in the development and manufacturing of nasal and pulmonary drug delivery devices, to develop a unique, customized dose indicator for delivery of the drug. The partnership endeavored over the course of six years to come up with an effective solution.
Just what the doctor ordered
The end result: The team came up with an innovative aerosol-based delivery method intended to offer an alternative to conventional spray pump-based systems. Zetonna is a CFCfree treatment formulated with environmentally friendly hydrofluoroalkane (HFA) propellant in a pressurized metered dose inhaler (pMDI). Each canister of the nasal aerosol contains enough medicine for 60 metered actuations, equivalent to one month of treatment.
The center of the delivery system is Aptar Pharma's Landmark dose-counting actuator. Landmark counts downward and provides the patient with a visual color and numerical reference when it is time to refill the prescription, before the container becomes empty and potentially catches the patient by surprise. The Landmark mechanism (which consists of just four components) features displacement-driven counting and operates independently from the patient's actuation force; the counting has no impact on valve actuation. The Landmark color-coded, numeric display is designed to provide clear indication of the actuator's remaining doses. The compact, robust design of the device includes a protective plastic cap, which guards the nose tip from being affected by dust and other contaminants. The cap is permanently attached to the actuator to prevent it from getting lost. Four different stem-valve diameters ranging from 2.8 mm to 3.18 mm are available.
Alternative options
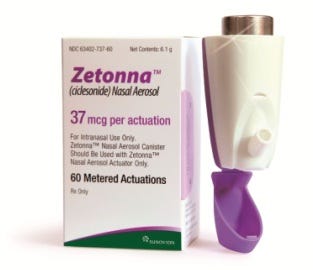
Sunovion Pharmaceuticals Zetonna
Sunovion Pharmaceuticals also offers a version of its ciclesonide treatment intended for allergic rhinitis sufferers in an aqueous spray format, under the brand name Omnaris. Many physicians treating patients with allergic rhinitis often find that their preference in allergy drugs, and the packaging and delivery methods available to them, can vary from person to person, and one size does not fit all.
According to William Berger, M.D., an allergist in Mission Viejo, CA, and a clinical professor of allergy and immunology at the University of California Irvine, says patients frequently reach out to their doctors looking for alternative allergy relief methods.
"Nasal corticosteroids like Zetonna are efficacious treatments for allergic rhinitis," he says. "Now that we have the option of prescribing either an aqueous or dry delivery formulation of this steroid, we have another way of treating our patients' discomfort with allergies, which may help them to achieve greater satisfaction."
Michael Blaiss, M.D., an allergist and clinical professor at the University of Tennessee, says that a new packaging or delivery format like the Zetonna actuator can actually make a difference in patient adherence.
"Patients are more likely to stick to their treatment regimen if they are prescribed a medication that fits their individual needs, so having different options for patients continues to be important," he says. "With the availability of the Zetonna nasal aerosol, patients will have an additional treatment option available to help manage their symptoms of allergic rhinitis year-round."
Hitting the mark
The Landmark actuator, customized for the Zetonna project, helps Sunovion Pharmaceuticals comply with U.S. Food and Drug Administration (FDA) requirements. The agency has issued recommendations that pharmaceutical manufacturers ensure that their delivery devices for new pMDI products integrate a dose-counting device. Metered-dose inhalers have been on the market for approximately 50 years; however, for most of that history, the devices frustrated patients who had no reliable way of knowing when their medication was about to run out. To better serve patients and avoid subjecting them to gaps in medication, the FDA issued a guidance in 2003 outlining how pharma manufacturers should incorporate dose-counting mechanisms in their delivery devices. To meet those goals, Aptar Pharma developed the Landmark actuator to be compliant with functional and technical requirements laid out by the FDA.
In addition to being selected by Sunovion Pharmaceuticals for the Zetonna delivery system, the product has received a design excellence award from the European Aerosol Federation (FEA) for its innovative design.
Successful partnership
According to Ted Raad, vp of sales and marketing for Sunovion Pharmaceuticals, the introduction of the Landmark actuator was crucial in the creation and launch of the Zetonna nasal aerosol itself.
"Aptar Pharma's Landmark indicator has played a key role in the development of Zetonna," he says. "The launch of Zetonna nasal aerosol in the United States is an important milestone for Sunovion, and it provides another treatment option for the millions of patients suffering from allergic rhinitis."
According to Pierre Carlotti, vp of marketing and communication for Aptar Pharma's Prescription Div., the collaboration between the two companies helped bring about creation of a successful, effective delivery tool.
"We are particularly proud to have developed this customized device for Zetonna," Carlotti says. "The collaboration between Aptar Pharma and Sunovion Pharmaceuticals is a source of great satisfaction."
Solid results
Sunovion Pharmaceuticals put Zetonna in its new delivery system to the test in a two-week study of 327 allergy sufferers. According to the company, test subjects responded positively to the drug and the new actuator. People suffering from both seasonal allergic rhinitis (SAR) and perennial allergic rhinitis (PAR) reported significant abatement of their nasal symptoms and improved quality of life (activity, sleep, nose and eye health, emotional function and other areas).
Sunovion Pharmaceuticals launched Zetonna in the U.S. at the end of July. The product's introduction was timed to be in patients' hands ahead of the autumn allergy season.
.
About the Author(s)
You May Also Like


