Here’s how artificial intelligence (AI) technology for inspection systems can help safeguard the efficiency and profitability of your solid oral dose packaging line.

What do pills, defective products, and pharmaceutical packaging have to do with one another? While they are three different topics, all of them are vital for delivering high-quality medicine. Defective pills or capsules can contribute to wasted packaging costs when damage causes an entire package to be rejected.
Let’s take a closer look at how you can prevent these issues from negatively impacting your business.
The high price of pill or capsule defects.
Pharmaceutical product defects are simply any flaw in the pill’s casing. This can include anything from a broken seal to a chip in the side of the capsule. These types of defects can cause all sorts of problems, but two of the most common issues are with either the medication itself or with the packaging.
Medication Defects: When pills or capsules have a defect, it directly impacts the pill’s contents. If medication leaks out because of damage, or if there is an error in dosage because of missing or broken pills, this can cause issues for patients who rely on these medications to work as prescribed.
Additionally, solid oral doses that have broken seals can lead to the pill’s contents becoming exposed to outside contaminants. This not only makes the medication less effective, but it could also lead to bacterial contamination and serious health risks for patients.
Packaging Defects: Defective products can ruin pharmaceutical packaging if the product defect causes the medication to leak out and contaminate the packaging and/or the rest of the products in the package. This not only creates extra waste but could also put patients at risk for serious health complications.
Compromised containers can present a financial risk for manufacturers. In any manufacturing environment, high scrap rates should be avoided. For pharmaceutical packaging, a product defect that causes an entire package to become discarded can lead to serious financial losses for manufacturers — both in terms of lost materials (products and packages), and in the lost time to identify and correct the anomaly.
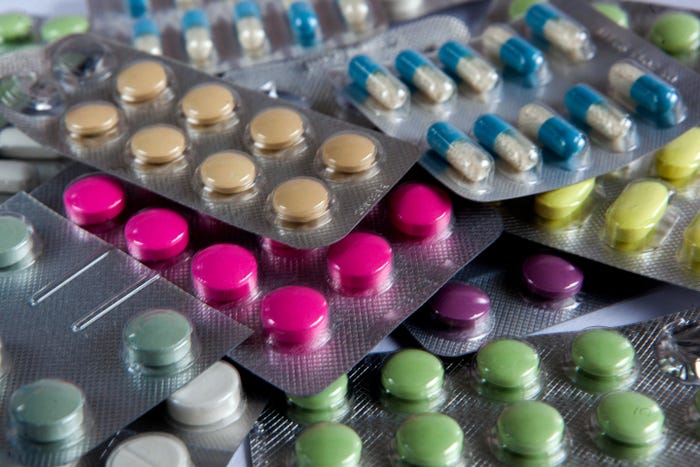
How can pharmaceutical packaging companies avoid defects?
To avoid defects in pills or capsules, pharmaceutical companies must take precautions when manufacturing and packaging their products. This includes following standard operating procedures, using quality control measures, and employing a vigilant workforce. By taking these steps, companies can help ensure that solid oral doses are manufactured correctly and without defects.
Many packaging lines use machine vision or, more recently, automated artificial intelligence (AI) inspection systems to identify defects. These systems can identify product or packaging defects early in the manufacturing processes, ultimately increasing the overall efficiency of pharmaceutical production.
Pharmaceutical companies must have a system in place for dealing with defective products. This includes having protocols in place for identifying and correcting any errors as quickly as possible. By doing this, pharmaceutical companies can help minimize the impact that defective products have on their packaging lines and ensure that patients receive the safe, effective medications they need.
AI pill inspection systems can be an effective way to help pharmaceutical companies avoid defects. AI techniques such as deep learning can be advantageous compared to traditional image processing techniques for images with changing environmental conditions. For example, AI is generally robust to different lighting intensities, whereas machine vision will require new rules-based programming. Beyond the technical factors, here are three reasons why you should consider AI inspection for your packaging line:
1. Increased production speeds: With AI pill inspection, it’s possible to increase the speed at which solid oral doses travel through a packaging line. This could dramatically decrease manufacturing time and the number of pill defects that occur.
2. Better quality control: AI pill inspection can help pharmaceutical companies maintain better quality standards for their pill packaging. Automated systems can identify defects more quickly than humans, which means defective pills aren’t packaged before they can be identified as errors. This helps reduce manufacturing costs by minimizing rejects.

3. More accurate product counts: AI inspection can help manufacturers maintain the correct number of counts in each container by keeping track of pill orientations and pill positions within a package or bottle. This ensures consistency not only across packages but also throughout each individual one, which leads to more effective pharmaceutical packaging overall.
Ensuring efficiency in pharmaceutical manufacturing and its respective packaging process should be a top priority for manufacturers. By taking steps to implement AI inspection systems and other quality control measures, pharmaceutical companies can help reduce the risk of defects and errors, as well as protect against financial losses due to defective products or packages.
About the Author(s)
You May Also Like




