Infection prevention, patient adherence, self-administration, and increased drug production are key drivers of the expected 6.4% yearly growth of unit-dose containers.
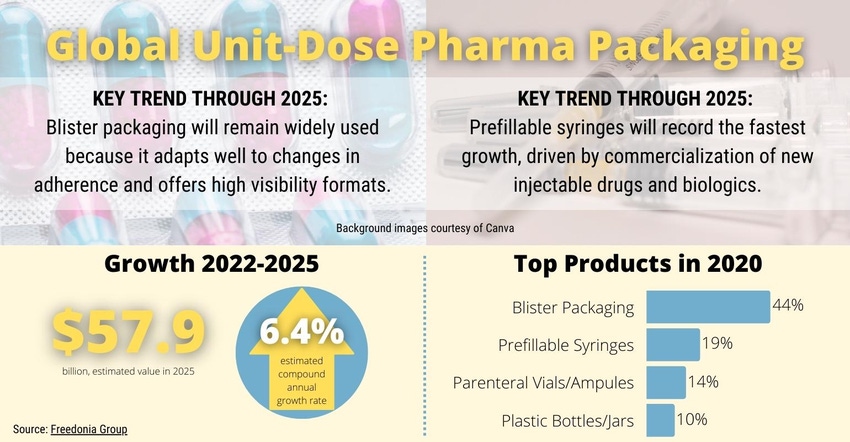
Demand for unit-dose drug-delivery devices and packaging will rise 6.4% annually to nearly $57.9 billion in 2025, according to a new Freedonia Group analysis, “The Global Unit Dose Pharmaceutical Packaging Report.” Blister packaging for solid dose applications will lead the global market, while prefillable syringes and parenteral vials will see the fastest growth.
Improving patient compliance through packaging designs is a major trend supporting the unit-dose market. This will accelerate the use of blister and strip packs, as well as other unit-dose containers. Clinical drug trials will also boost growth prospects for unit-dose packaging systems that promote compliance and recordkeeping.
The study anticipates a boost in applications for auto-retractable prefillable syringes, pen adaptable cartridges, and high-barrier parenteral vials throughout the global medical community in response to the need for better infection prevention systems.
Competition drives blister growth.
The report indicates that intensifying generic and private label competition is prompting drug makers to upgrade containers to influence buying decisions of consumers and medical providers — a boon for unit-dose blister packs.
“Generic and private label competition is increasing because of downward pricing pressures imposed by public and private health insurance providers,” says Bill Martineau, Freedonia analyst. “Also, the number of generic producers and distributors are increasing while the number of proprietary drug producers and distributors are decreasing slightly due to mergers and acquisitions.”
According to Martineau, efforts to improve the aesthetic and convenience properties of pharmaceutical packaging applies more to over-the-counter drugs and supplements sold in retail stores. On the other hand, improvements in ethical drug packaging primarily involve infection prevention and ease-of-delivery features.
New therapies, vaccines call for unit-dose syringes.
The study predicts that new and biosimilar therapies for cancer, viral disorders, and other debilitating diseases will drive demand for unit-dose prefillable syringes and parenteral vials. According to Martineau, these drugs are almost always packaged in unit-dose containers.
Moreover, ongoing improvements in syringe design and safety has led to a rise in therapies approved for self-injection. This, in turn, will accelerate demand for unit-dose prefillable syringes and parenteral vials. Martineau shares that self-injection therapies are usually packaged in either single-dose syringes or multi-use devices with cartridges.
A heightened focus on infection prevention in parenteral drug delivery systems used in hospitals, clinics, physicians’ offices, and nursing homes will also contribute to the growth of unit-dose syringes and parenteral vials.
Finally, demand for these unit-dose formats will increase in response to the growth of global preventive medicine, which requires single-dose injectable vaccines for diseases such as COVID-19, influenza, meningitis, and shingles.
About the Author(s)
You May Also Like




