March 11, 2015
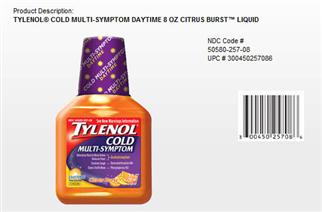
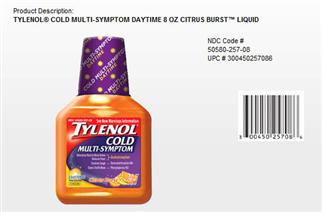
one of the Tylenol Cold packages being recalled
In consultation with the U.S. Food and Drug Administration (FDA), McNeil Consumer Healthcare, Division of McNEIL-PPC, Inc., is recalling, from the wholesale and retail level, three TYLENOL® Cold Multi-Symptom liquid products in order to update the labeling for these products.
McNeil Consumer Healthcare initiated the recall after an internal review revealed that information about the presence of alcohol from flavoring agents was noted as an inactive ingredient listed on the package, but not on the front panel of the product. Certain flavoring agents contribute small (< 1 percent) amounts of alcohol.
This is a wholesale and retail level recall and is not being undertaken on the basis of adverse events. No action is required by consumers or healthcare providers and consumers can continue to use the product.
Consumers with questions should call the FDA's Consumer Care Center at 1-888-222-6036 (available Monday-Friday from 8 a.m. - 8 p.m. ET and Saturday - Sunday, 9 a.m. - 5 p.m. Eastern Time.)
The NDC codes for the recalled products can be found on the product front panel.
Source: FDA
About the Author(s)
You May Also Like


