Demand for the COVID-19 vaccines severely exceeds current supply. So, in a move similar to the unprecedented collaboration between Sanofi and Pfizer, now Merck has stepped up to help get the Johnson & Johnson vaccine to the market quicker.

Top headlines in the community of pharmaceutical packaging keep you up to date on what’s happening now.
Merck Helps Produce J&J’s COVID-19 Vaccine
Demand for the COVID-19 vaccines severely exceeds current supply. So, in a move similar to the unprecedented collaboration between Sanofi and Pfizer, now Merck has stepped up to help get the Johnson & Johnson vaccine to the market quicker.
Merck is using money it received from the Biomedical Advanced Research and Development Authority (BARDA) to adapt two existing manufacturing facilities to “to accelerate manufacturing efforts for the vaccine and enable more timely delivery and administration.” BARDA is a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the US Department of Health and Human Services (HHS).
The plant upgrades include vial packaging (fill-finish). One facility will also be set up to make the vaccine, as well as package it.
The number of doses Merck expects to produce was not shared but they are expected to be released towards the second half of the year, reports The Washington Post, “when the threat of variants could loom larger, requiring the manufacture of booster shots, and also when greater political attention will turn to supplying the developing world with vaccine.”

Training Device Helps Personnel Administer Opioid Treatment
A leader in addiction medicine in Northern Europe, dne pharma is partnering with Aptar Pharma and one of its companies, Noble, on a nasal drug delivery training kit for Ventizolve opioid overdose treatment as part of a broad patient onboarding and awareness program in outreach and drug treatment centers across the Nordics.
Ventizolve is packaged in Aptar Pharma’s Unidose Liquid System, which is a ready-to-use, one-step nasal device that delivers a single dose quickly, easily, and reliably — and without the need for administration by a trained professional.
The training device, developed by Noble, copies the form and function of the Unidose device. But it also has a novel twist reset function, so users and caregivers can practice administering a dose, so they are ready when confronted with an actual emergency.

Chad Tyler Leads Sales at Pharmaworks
Pharmaworks, part of ProMach Pharma Solutions, has appointed Chad Tyler to the position of Director of Sales, responsible for leading the team that brings Pharmaworks’ blister machines and other packaging systems to pharmaceutical, nutraceutical, and medical device manufacturers and contract packers around the world.
Tyler has 20 years of experience in pharmaceutical packaging, manufacturing, engineering, management, and sales. Most recently, he was chief technology officer at Jekson USA Inc. Before that, he was at Optel Group for 10 years, holding positions of increasing responsibilities, including senior director of global corporate accounts, as well as European Program Manager, based in the UK.
Partnership Ensures Safe Transport of Vaccines in Korea
SkyCell, a leading manufacturer of data-driven temperature-controlled containers for the pharmaceutical industry, has partnered with Korean Air Cargo. Cold-chain maintenance for vaccine transportation is a significant challenge for the global logistics industry, and together SkyCell and Korean Air will work together to ensure vaccines reach patients safely.
Through this agreement, Korean Air will offer SkyCell’s hybrid containers to clients, providing them with a safe and sustainable solution to protect temperature-sensitive products.
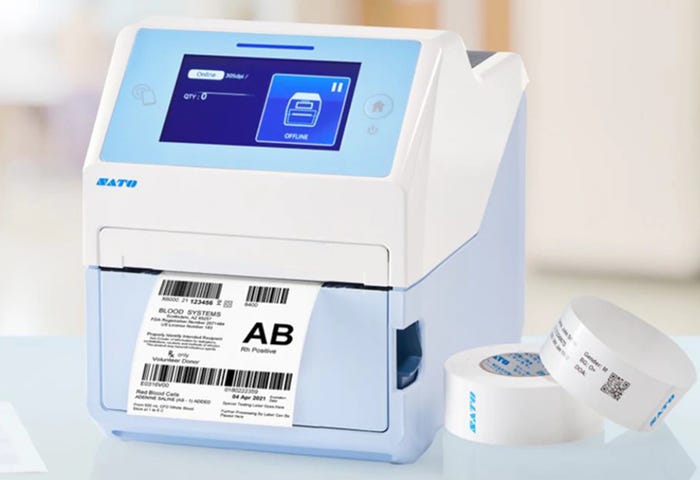
New Desktop Printer Creates Healthcare ID Labels
The CT4-LX-HC compact desktop printer from Sato can create healthcare identification labels throughout the supply chain, including at the point of care, such as for specimen labels. As extra safety precautions, it has an anti-microbial casing that is disinfectant wipe-down-ready to ensure hygiene when multiple users need to use its 4.3-inch touchscreen display in the same workplace.
About the Author(s)
You May Also Like




