December 21, 2015
Pharmaceutical and medical device manufacturing companies are enlisting their packaging departments to protect much more than product integrity. Profitability, brand reputation, and even the environment are being preserved with the help of packaging.
Minimizing risk is pretty high on any professional's list. For healthcare product packagers, avoiding risk has usually meant selecting materials and machinery that can be used to best protect drugs and medical devices from traditional hazards like moisture and loss of sterility.
In today's healthcare market, however, much more than product integrity is at risk for healthcare product manufacturers. "Our industry is facing unprecedented challenges," notes Rich Hollander, senior director of packaging services for Pfizer and a member of PMP News's editorial advisory board. "[There's] loss of exclusivity, increased regulatory and medical scrutiny resulting in fewer new product approvals, payers not willing or not able to pay, counterfeiting on the rise, and, of course, the need to reduce costs. The need for innovation in technology has never been higher."
Controlling cost has always been key for packaging professionals. But controlling costs may not necessarily mean reducing costs—it may mean spending capital in ways that reduce product and company risk.
For Stephen Hess, executive director of packaging technology for Merck and Co., Inc., cost-effectiveness is a definite priority. "The industry is really focusing on controlling costs without adversely affecting quality," says Hess, who also serves on PMP News's editorial advisory board.
For instance, one healthcare packager responding to PMP News's annual Healthcare Packaging Trends survey says that his company struggles to "balance cost versus risk." Other respondents wring their hands over "ever-rising packaging costs."
Industry suppliers and contractors are acutely aware of the concern. "I think the entire pharma packaging industry is feeling the cost pressure," says Kevin Carter, sales executive for RxPak, McKesson's contract packaging division. "I have heard that customers are evaluating packaging mediums, such as bottle versus blister, compliance versus bottle, and unit-of-use versus bulk. They are focusing on direct costs as well as evaluating delivered costs through the process."
Adds Stuart Baker, vice president of sales, for Advanced Poly-Packaging Inc. (Akron, OH): "The biggest challenge facing our customers is quite basic: trying to find ways to cut costs without jeopardizing validation requirements or overall quality."
Focusing on quality may, in fact, offer solutions for cost containment. "Cost pressures are affecting the entire industry," says Akan Oton, global director of marketing for packaging services and clinical supply at Catalent Pharma Solutions. "Companies are focusing on operational excellence and reaching Six Sigma. For instance, they are increasingly concerned about out-of-spec components slowing things down on packaging lines. They are also demanding on-time delivery," he says.
As a result, "Packaging decisions are made based upon demonstrated added value," adds Hess. "In the past, we may have made a decision to go to a wallet because someone liked the way it looked. Now, we work to demonstrate the value of packaging from the perspective of the patient, payer, and prescriber."
And other risks plague healthcare and therefore product manufacturers. The global supply chain presents products with diversion risks as well as distribution hazards. Medical errors and noncompliance continue to threaten consumer health. And while child resistance has always been a concern for packagers, the growing number of sophisticated at-home treatments for chronic health conditions is also emphasizing the need for patient-friendly packaging and labeling.
Observes Remco van Weeren, PhD, senior vice president, marketing and technology, for Bilcare Inc.: "Companies are looking for help for what I like to call the five Cs—cost, communication, compliance, convenience, and counterfeiting."
Reducing environmental risks is also being added to packagers' checklists. Wal-Mart's interest in sustainability, for example, is forcing a second look at materials and processes.
Today's packaging professionals are increasingly being asked to employ packaging that protects healthcare products and patients from these risks and more. They are asking themselves, "What are the risks to my products and therefore my company's bottom line? How can packaging mitigate those risks?"
Inefficiency and waste
Pharmaceutical and medical device manufacturers are working to minimize a number of economic risks. Inefficient, wasteful production lines are certainly prime for change. But solutions vary, depending upon company and product. One survey respondent explains that he has to juggle high numbers of low-volume SKUs. Another welcomes emerging solutions for line speed. One packager is spending a lot of money on a labor-intensive inspection process, while others are working to automate lines and to employ vision technologies for filling accuracy.
One commonality is apparent—packaging lines have to be immediately responsive to company and industry needs for high-quality, cost-effective packaging. "Our customers, like everyone, are faced with decreasing lead times and financial constraints," says Jonathan Calderwood, global marketing manager for clinical packaging supplier Almac Group. "Specific client challenges are many, but most relate to process improvement and streamlining operations within the supply chain."
For instance, lean manufacturing initiatives are taking on greater importance in the pharmaceutical industry, reports Nick Fotis, director of Cardinal Health's Packaging Technology Center. "The inherent contradiction of global sourcing, which drives higher inventories, with lean manufacturing, which demands lower inventories, is placing conflicting demands on the packaging engineer," he says.
"Big pharma is emphasizing lean manufacturing and Six Sigma," adds van Weeren of Bilcare. "They are looking for assistance with improving inventory management and additional services that minimize risk at their end."
To meet these demands, packaging departments have to be equipped in order to turn product campaigns around quickly and consistently. And that equipment has to be flexible and dependable. "We are increasingly looking for equipment solutions that exhibit SMED (single-minute exchange of dies), printing technologies that have instantaneous changeover with no messy ink cleanup, and standardized packaging systems," says Fotis. "Additionally, we are increasingly justifying many, smaller machines instead of the single, dedicated lines of the past."
To increase packaging productivity and accuracy, Automated Packaging Systems (Streetsboro, OH) is building industrial PCs and high-speed communications into its Autobag packaging systems. "With such advanced technology, we are able to integrate with a customer's manufacturing, production, and fulfillment databases for higher productivity and higher accuracy packaging," says Tim Groff, director of marketing. "The most significant advancements in packaging technology this past year have been related to systems integration, where our baggers, printers, and packaging systems are communicating with external computer control systems via DeviceNet or high-speed Ethernet. We utilize intelligent process control to run the packaging operation and communicate back to a central database or programming control center."
This emerging trend of enhanced system integration has been most prominent in the pharmacy fulfillment market, where validation has become a critical issue, Groff adds. "From individual order fulfillment to unit-dose packaging, the importance of validating package contents and patient delivery information has become a unique opportunity to enhance the packaging system as a key link between distribution and delivery. We have worked with systems integrators to design custom packaging solutions that use bar code scanners to read and verify the pharmaceutical that is being packaged, the personalized literature that is included, and also the patient information on the outside of the package. Each of these three important steps is verified by database communications and if any one component is not verifiable, the package is flagged as an error."
For web printing, CSAT America LLC (Louisville, CO) has updated its technology to a 1200 × 1200-dpi resolution computer-to-print system for in-line and off-line applications, reports Joseph Buono, sales manager. "We are providing alternatives for printing flexibility to companies such as J&J, Tyco, and Merck, [giving them] the ability to print with frequent changeovers and limited staffing."
As companies add to their product lines with brand extensions and even product acquisitions, packaging professionals look for cost-effective ways to increase capacity. Flexibility, quick changeover, and versatility in machinery are often sought. Walt Langosch, president of ESS Technologies Inc. (Blacksburg, VA), reports that new product introductions and product acquisitions are forcing many manufacturers to combine new products with existing products on existing lines. Integrating these products while maintaining efficiency and productivity and dealing with additional changeovers can be challenging. To address this trend, "ESS provides a production line audit/evaluation to determine the best and most affordable method to expand the capabilities of the production line while increasing flexibility, reducing changeover time, and increasing output," says Langosch. "This often involves enhancements to material handling and controls and incorporating flexible automation like robotics for several functions relating to machine loading and unloading, product collation, and end-of-line automation. The further addition of inspection and vision systems increase the output and product quality."
Baker adds that Advanced Poly-Packaging developed the T-1000 medical bagger because "medical packagers needed a cost-effective way to increase their productivity. We modified our most popular machine and developed preopened Tyvek bags on rolls for a solution." The firm also developed the T-375 tabletop bagger/printer, which can often replace large bagging systems that are difficult to operate and expensive to maintain. "The machine's next-bag-out printing capability allows packagers to fill the bag that was just printed. Other machines print bags that are several cycles behind the one that's being filled. The release of the T-375 tabletop bagger brings another form of automation to pharmaceutical bagging."
To get companies' sterile packaging lines delivered and running sooner (thereby saving on development time and cost), IMA North America (Bristol, PA) has devised its MAC line. "We have compressed a sterile-filling line into a footprint that is 20% of a traditional line," explains Warren Roman, president of IMA North America. "We believe we can cut the time from machine order to full validation from 2–3 years to 6–8 months." Sized to fit on a truck, the modular line can use any filling system and can be isolated easily. It runs about 100 vials per minute. "We have found that with a slower, smaller line, cleanup between batches is faster. Potential users have to consider the total amount of time needed to set up and clean up lines," he says. While Roman believes that it will be popular for clinical biotech packaging, he says that it can be scaled up for production.
The most significant machinery trend that Multivac Inc. (Kansas City, MO) has seen is the growing support for open, modular architecture control (OMAC) technologies, reports Jerry Hirsh, marketing manager for Multivac. "OMAC standards enable users to have more freedom in selecting vendors and products, resulting in more competition and therefore better economics for pharmaceutical and medical packagers."
The OMAC packaging work group will be working with the OPC Foundation to demonstrate an integrated packaging line during Pack Expo Las Vegas 2007 this October. Connect-and-Pack guidelines from the group "provide a common look and feel to each of the [following] packaging line functions: control, HMI, MES, and ERP," explains the group's Web site. "This commonality provides the benefits of reduced engineering cost, reduced training cost, and improved asset utilization from the resulting transparent access to all relevant machine data." PackML and PackTags, part of the guidelines, "provide a common machine language and defined set of packaging tags that enable the common look and feel."
Even clinical trial packaging operations are being asked to run more efficiently and flexibly. Calderwood of Almac Clinical Services, says that in the growing market for blinding of comparator products, "There is a need for flexible lines that can match the batch requirements from hundreds to millions of capsules. Our latest systems are designed primarily to tackle bottlenecks in clinical packaging and provide security in terms of full automation and control of fill. Our modular encapsulation system has been employed primarily to reduce the lead times associated with blinding and overencapsulation of comparator products."
Almac has also introduced what Calderwood calls "the first fully automated label verification system that is unique to the industry in terms of being able to read fixed and variable content, a feature designed primarily for clinical supplies where randomization is central to many packaging activities."
Carter of RxPak says that he has seen that companies are maximizing their own capacities and seem to be investing less capital in new equipment. "This could be a positive factor for the contract business as a whole over the next one to two years."
Materials providers are also working toward providing more value at the behest of packagers. "There is an increased awareness of the role of packaging in product security, patient compliance, and other key issues, which makes packaging a serious ‘value-add' in the business process of delivering drugs," says Narendra Srivatsa, business development manager for Cortegra. "This was not as prevalent even five years ago."
Films provider Klöckner Pentaplast (Gordonsville, VA) is working to speed time-to-market and control costs with its Pentapharm BlisterPro program. "Through this software program, we are able to evaluate the most cost-effective film options for packaging their products," says Daniel Stagnaro, pharmaceutical films business manager. "By evaluating several materials simultaneously without a physical sample, we can reduce time-to-market, minimizing machine trials and tooling costs."
Catalent has invested in state-of-the-art vision inspection and printing systems for its printed components such as labels, cartons, and inserts. "Running such components is a critical part of today's packaging lines," says Oton. "If printed components don't work well on the line or the paperboard isn't appropriate for a particular line, companies run into real problems. Because we print components and run packaging lines, we are able to design and test our printed components at typical line speeds to ensure that they are runnable."
Counterfeiting and diversion
Several respondents indicate that RFID, anticounterfeiting technologies, and security packaging will play vital roles in their programs.
Drug pedigree requirements will have a significant effect on industry. "While pedigree and mass serialization are among technologies that may help deal with the issue of counterfeiting and diversion, we must realize that they have the potential to really impact our product packaging and costs," says Hess. "We want to make sure that we do whatever is possible to fight counterfeiting and illegal diversion. We are all working on how to comply with California and with potential federal legislation under the FDA revitalization act. We need to be sure that whatever technologies are adopted really have an impact because they will drive significant costs." "
Adam Sasso, director of communications for The Challenge Printing Co. (Clifton, NJ), says that his firm is engaged in a number of drug pilot programs for both high-frequency (HF) and ultra-high-frequency (UHF) RFID tags. "It is expected that the results of these pilots will drive item-level tagging among manufacturers seeking to comply with the fast-approaching January 1, 2009, California ePedigree mandate and that even more pilots will be undertaken in the coming months."
Advances in RFID technology are making it more appealing for a wider group of applications. "While cost reductions have garnered significant press, little has been published regarding the way Gen2 near-field tags have overcome technical hurdles in the presence of liquids and metals in the UHF or 915-MHz space," says Sasso. "Although this could not have been foreseen five years ago, given what is likely to be a sustainable cost advantage over HF, this development may tip the eventual widespread adoption of RFID for item-level tagging in UHF's direction."
Catalent supports RFID, but it also sees a strong role for 2-D bar codes. "We believe there is a place for both. Through our partnership with Secure Symbology Inc., we are able to offer a turnkey solution for bar code serialization," says Oton. "SSI's algorithms provide for a reliable track-and-trace solution that can be implemented today versus RFID. We have demonstrated the robustness of its equipment in manufacturing environments."
Challenge Printing also sees an increase in the use of overt and covert authentication and antidiversion devices in product labeling to better protect patients and pharmaceutical manufacturers.
Srivatsa says that Cortegra continues to incorporate new technologies in its RxTrackNSecure security offering. "We are getting close to announcing a technology in our RxTrackNSecure portfolio that will enable patient safety to a higher level than today."
Adulteration
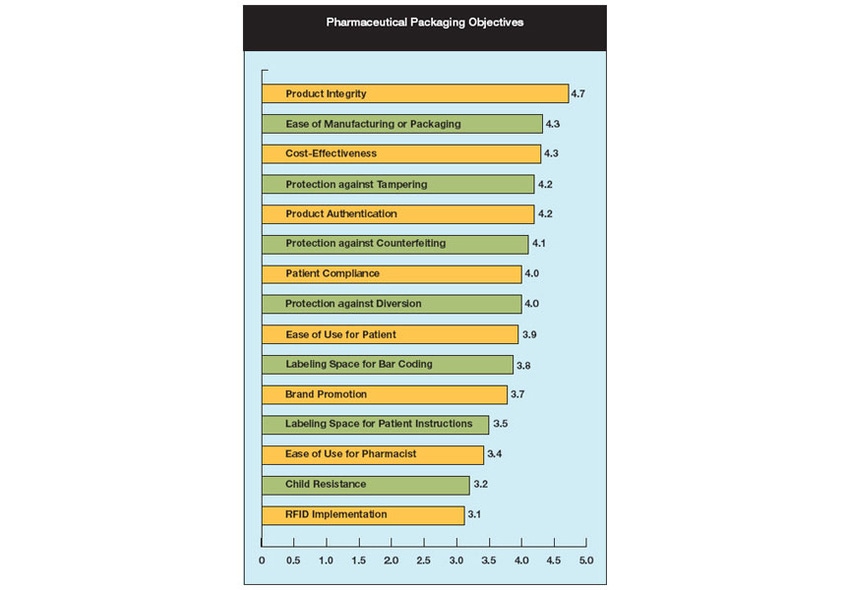
Adulterated is FDA's catchall term for contaminated, unsterile, unsafe, spoiled, or expired products. Avoiding such conditions continues to be the top priority for both pharmaceutical and medical device packaging professionals. Survey respondents put product integrity as their number one objective: on a scale of 1 to 5 with 5 being very important, medical packagers rated product integrity at 4.8, while pharmaceutical packagers put it at 4.7. (For other priority rankings, see Figures 1 and 2.)
To ensure product integrity, packaging professionals are keeping a close eye on material quality. "The main healthcare trend has been the tightening of quality standards past established AQL and more toward zero defect," explains Lawrence Blake, director, marketing, for Alcan Packaging–Plastics Americas (Pennsauken, NJ). "This additional attention to quality detail means that all suppliers have to control their manufacturing standards even better and continue to add back-end quality systems to test and validate good product."
Packagers are also looking for higher barrier. Beacon Converters' latest technologies focus on improved barrier properties to moisture, UV light, oxygen, and most recently carbon dioxide. "Customers are demanding higher-performing products, but don't necessarily want foil as a component," says Kathleen Daly Mascolo, vice president, director of sales and marketing. "The increase in biorelated products has created an increased need for control of gases that never used to be of concern, such as carbon dioxide. The food industry has been working with CO2 barriers for a long time, but it is relatively new to medical. Most products don't even have CO2 measured. The use of dry ice for shipping and short-term storage has created this need, as dry ice is frozen CO2 and can displace the gases inside a package during transit. CO2 and high humidity can be corrosive." (Read about Beacon's anniversary on page 20.)
For EtO-sterilized products needing moisture barrier, Alcan Packaging– Medical Flexibles (Chicago) has been working on moisture-absorbing foil laminations and incorporating those structures into a foil pouch with a Tyvek header, says Jesse Blake, marketing manager, describing DesiShield and DesiVent, respectively.
Klöckner Pentaplast has created multiple laminated and coated structures that place the barrier component closer to the product, increasing barrier performance without increasing cost, says Stagnaro, pointing to products such as Pentapharm Aclar S03 and Pentapharm alfoil E S03 films.
The recent revision of ISO 11607 continues to refine the science of medical packaging. Medical device manufacturers have more details on how to develop, qualify, and validate packaging, but they ultimately decide their own course. For instance, a number of potential test methods are listed, but not mandated. Packagers need to choose the methods most appropriate for their products and companies and then validate those methods in their own labs.
"The approach to achieving compliance to ISO 11607 will vary among medical device manufacturers, but in each case the approach chosen should be supported by an appropriate rationale to be included in the documented package validation protocol," writes Dhuanne Dodrill, president of Rollprint Packaging Products Inc., in PMP News's July 2007 Regulatory Focus. "The choice will be dependent on corporate risk policy and economic considerations."
According to the revision, sterile packaging manufacturers are shouldering more responsibility in validating the manufacturing processes they use to make the preformed sterile barrier systems they produce for device manufacturers.
DuPont Medical Packaging has released materials documenting that "Tyvek conforms to all of the ‘shall' statements in clause 4, the materials section of ISO 11607-1:2006," reports Curt Larsen, principal of Spartan Design Group and a senior consultant for DuPont as well as a PMP News EAB member. "It is available for download from our Web site and will help the MDMs and SPMs in their compliance efforts, too."
Sterile packaging manufacturers are providing the support. Randy Troutman, technical director, Oliver Medical (Grand Rapids, MI), says that the newly stated "converter validation requirements are driving process improvements." In addition, medical device manufacturers are asking for "better, more-robust data" from converters as well as vendor-managed inventory and dock-to-stock programs. Oliver Medical has developed its Advanced Technical Services to support these requests and others, such as validation support. It even assists customers not using its products. "With support upfront, customers feel confident switching to our materials if they make sense," says Troutman. "As former project manager for global packaging development at Smith and Nephew, I know that better data will tell the story."
Alcan Packaging's Neenah Technical Center (Neenah, WI) continues to expand to provide customers with extensive analytical and laboratory resources, reports Rick Merical, director of R&D for Alcan Packaging– Medical Flexibles. "We are seeing customers more and more utilizing our capability for extractables and leachables testing and premarket launch accelerated-aging studies that create peace-of-mind during product development," he says. "The recently created Innovation Center as part of the Neenah Technical Center also brings added value to product development. These capabilities go beyond the traditional purely product development process to the entire value stream of services necessary to ensure that quality, sustainability, and performance attributes are completely built into new products and technologies."
Noncompliance
Carter says that he sees a large drive in the industry for compliance-style packaging. "We are working with several partner companies on new package concepts and the automation of those packaging systems," he says. "Some are using the now-traditional walleted solutions. Some newer concepts involve injection-molded components combined with blister technologies, and in others, stand-alone injection-molded systems."
Daryl J. Madeira, director of marketing for Alcan Packaging–Contract Packaging and Specialty Cartons (Bethlehem, PA), has also seen an increased acceptance and affinity toward packaging solutions that improve patient compliance. "Both brand managers and packaging engineers are embracing packaging that facilitates better dosage calendarization, therapy instructions, improved brand connection, and authentication," he says. "Alcan has responded with primary and secondary design as well as infrastructure to provide all patient compliance packaging from concept to commercialization."
Oton from Catalent says that compliance packaging has to be cost-effective for market adoption. To that end, the firm developed its HingePak for efficient assembly in a single-step process without the use of specialty equipment.
Madeira also sees a continued sharp growth in drug-delivery mechanisms requiring unique, new-to-the-world packaging formats. Alcan responded to a unique drug-delivery need by developing a proprietary unit-dose tube packaging solution that can be sterilized by all commercial methods.
More and better labeling may also increase patients' understanding of product regimens. Possibly to that end, some respondents are turning to online printing for point-of-use packaging. Many are struggling to fit volumes of information onto already crammed labels and still maintain legibility.
"Compared with five years ago, packaging has moved toward more content on the same real estate or marginally higher real estate, which drives demands on print quality," says Srivatsa from Cortegra. "Font sizes get smaller, and yet have to meet the readability needs of a wide demographic group of different ages and other needs." He also points to FDA's recent labeling rule revisions requiring larger-format inserts.
Sasso says that Challenge Printing has seen a profound increase in FDA-mandated Medication Guides supplied to patients. "Based on presentations at a recent FDA public hearing, it is clear that unit-of-use packaging with manufacturer-supplied Medication Guides is, by far, the most effective method of getting this critical information into the hands of patients." This July, Challenge opened a new facility in Sanford, NC, increasing its capacity for producing printed pharmaceutical packaging components.
Van Weeren from Bilcare suggests an alternative term to compliance: persistence. "How do you make sure that patients take the drug for the long haul?" he asks. He laments that "more money is spent on getting new patients than helping existing ones."
Medical errors and misuse
Packaging and labeling have played increasingly important roles in easing product use, both by practitioners and patients. Designs will need to simplify procedures or at-home regimens and reduce the potential for medical errors or misuse. Child resistance will continue to be paramount for home-based therapies, especially since highly potent fast-acting drugs are emerging to treat a bevy of chronic conditions.
"The market is more focused on safer delivery systems and administration practices to reduce errors," reports Brian Lynch, senior product manager for BD Hypak, BD Medical—Pharmaceutical Systems (Franklin Lakes, NJ). "We have also seen a shift toward unit-dose packaging to better enable drug traceability in the marketplace. The use of prefillable syringes can help address each of these issues."
BD has three new self-injection technologies: a disposable auto-injector, a next-generation reconstitution system, and a solution for delivering high-volume, highly viscous drugs into the subcutaneous tissue. These products were developed to be intuitive and easy-to-use and to require a minimal number of steps.
Reduced hospital stays and increased home care are becoming more common, notes Fran DeGrazio, vice president, marketing and strategic business development, West Pharmaceutical Services (Lionville, PA). "We think this trend will continue, and it will drive our technologies in reconstitution. So many times a caregiver is tasked with handling a drug reconstitution and injection process. We want to make that process easier, more user-friendly."
West Pharmaceuticals' tools for reconstitution allow a lyophilized product in a vial to be directly connected to an IV bag, reconstituted, and infused. "The Vial2Bag product will ultimately replace the syringe with a needle. When you think about it—especially in a home-care environment—a product like this is really revolutionary. How many home caregivers would appreciate not being exposed to toxic drugs and not having accidental needle sticks? It fits on any IV bag, and it does not use a needle—a big safety benefit and a reduction or elimination of spillage," DeGrazio explains.
In the area of glass prefillable syringes, BD has developed two container closure systems that address healthcare market needs for security and patient injection comfort, says Janice Fajarito, product manager–BD Hypak. "BD Hypak PRTC provides a robust syringe-tip closure design that ensures secure tip cap attachment from handling and processing through administration. BD Hypak Physiolis is the sharpest staked prefillable syringe, which allows a 40% reduction in patient injection pain perception by enabling a 70% reduction in skin penetration force."
Some say that achieving adult-friendly child resistance for products to be used at home is a rare feat. "We continue to struggle with child-resistant blister packaging," says Hess. "These are typically our highest-complaint items and achieving the balance between child resistance and senior friendliness is very difficult."
Carter, who has witnessed the advent of more alternatives to traditional wallet-style compliance packages, says that interest in alternatives may relate to consumer responses to wallet systems related to child-resistance features and senior-friendly challenges. "Designers are looking for the proverbial magic-bullet compliance package, such as one that can meet the F=1 child-resistance requirements, attain success in senior-friendliness testing, and is well received by the typical consumer."
To ease bottle use, Alcan Packaging is launching its Securance line of caps, which include a one-piece child-resistant, senior-friendly squeeze-and-turn closure and an accordion-style child-resistant snap cap. "Both of these new closures can fit our new Secure Grip bottle," says Lawrence Blake. "The bottle has unique bottle dimensions and recesses that allow for easy handling."
Environmental pollution and depletion
Sustainability is gaining momentum. "I look to the sustainable packaging revolution to finally make its way into medical packaging," says Fotis. "Our customers are focused on infection prevention and cost reduction, but environmentally responsible packaging is so closely aligned with the medical professional's code of care for the patient, that all the industry needs is a leader to speak out and boldly articulate the hospital customer's expectations and sustainable packaging will take more of a center stage."
Survey respondents are looking for environmentally friendly materials and sterilization processes. In particular, some are looking for biodegradable plastics.
Merical from Alcan Packaging– Medical Flexibles says that many packaging "initiatives have been continuing with sustainability as a guiding influence." The firm's researchers are developing products with sustainability in mind, such as a high-barrier product using polylactic acid (PLA).
PMP News EAB member Hess points out that some approaches to sustainability may run contrary to other packaging functions: "We are unclear how we will be impacted, but can imagine that this could conflict with some of the patient-friendly packaging that we envision."
But sustainability does not just mean biodegradable, says Merical. "It can and does incorporate the entire aspect of packaging development and how we might be able to reduce our overall environmental footprint while at the same time providing innovative cost- effective quality packaging solutions."
Enhanced polymers permit the downgauging of some thermoforming film solutions by as much as 33%, while maintaining the same physical performance, he reports. In addition, "a full line of cold-seal platform products is permitting many customers to convert from traditional heat-seal packaging to cold-seal medical device packaging. The substantially reduced energy consumption of cold seal over heat seal plus the added benefit of source reduction from thinner packaging material demonstrates the aspect of how packaging can provide sustainable and value-added solutions."
Opportunities for using more-sustainable materials can be found at any packaging level. Pliant Corp. (Schaumburg, IL) introduced its Stratos family of ultra-high-yield films for pallet wrapping. Randy Scott, vice president of marketing and head of Pliant's sustainability initiative, reports that the materials "provide product manufacturers the opportunity to address corporate sustainability initiatives and reduce supply-chain costs without modifying the core product or primary packaging." Stratos includes 42- and 45-gauge films with the load containment properties of much thicker films. Material reduction can range from 15 to 50% depending on current film usage.
Limiting risk
A few years ago, Cardinal Health found a way to address many of these risks with packaging technology. The firm introduced its sterile-vent packaging system in the marketplace, recalls Fotis. "A winner of the Worldstar award [submitted by Multivac] a year ago, this packaging system places a Tyvek breathable vent in-line during package production. We expanded this packaging from our initial surgical drapes line to now include our procedural tray line. We have received a lot of positive attention and feedback on this innovation."
Boston Scientific Corp. has taken similar chances to address risks and come out ahead as well. It was recognized for its packaging in this year's Ameristar awards program, sponsored by the Institute of Packaging Professionals (IoPP) and presented at Packaging Summit. Boston Scientific selected a clear die-cut insert to replace a thermoformed tray used to package its cardiovascular fabrics, reports IoPP. "It is the first use of PETG as a die-cut, folded component. The advantages are lower component cost, improved sterile transfer in the operating room, increased throughput in manufacturing, and improved environmental impact."
About the Author(s)
You May Also Like




