March 11, 2015
By Joshua Russell, Mechanical Engineer, Automated Systems of Tacoma Inc.
Industrial manufacturing of injectable dr
100_6476.JPG
ug products presents very different and difficult challenges when compared to other industrial manufacturing applications. In order to enhance patient safety and ensure product quality the Pharmaceutical Industry has been implementing systems called Advanced Aseptic Processing (AAP) systems. Advanced Aseptic Processing systems utilize advanced forms of automation, robotics, machine vision, and separative devices in an effort to protect the pharmaceutical product from contamination. In the pharmaceutical industry these separative devices fall into two categories: Restricted Access Barrier Systems (RABS) and isolators.
Although driven by different reasons, Isolator-Barrier Systems and ANSI/RIA R15.06 (Safety Requirements for Industrial Robots and Robot Systems) have the same objective: restrict operator access to the most critical areas of the machinery. A RABS or isolator based robot system, when properly implemented according to ANSI/RIA R15.06 and current Good Manufacturing Practices (cGMP's), will provide a robot cell that meets the requirements for Advanced Aseptic Processing.
Advanced Aseptic Processing (AAP)
The Parenteral Drug Association (PDA) describes an Aseptic Process as, "The process f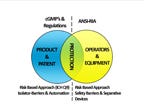
ANSI-RIA_cGMP Protection Chart.jpg
or manufacturing sterile products by which microbiological contamination is eliminated from the product and product contact surfaces protecting the product from sources of contamination" (PDA Technical Report No. 44, 2008). The challenge for pharmaceutical drug manufacturers is to ensure that the products they manufacture are made in a manner that precludes microbiological contamination. This is especially important for injectable or parenteral drugs, which are the products having the highest risk, because the injection bypasses all barriers nature has provided for the patient.
Traditionally, an operator within an open, ISO 5 cleanroom environment performs sterile drug manufacturing using automated or semi-automated machinery, or is even done manually. Although operators within the cleanroom environment wear sterile cleanroom garments, they remain the greatest contributor to cleanroom and product contamination. A study by Whyte (Whyte, 1998) showed how activity affects particle generation rates with people wearing cleanroom gowning for particles 0.5um in size:
•Sitting motionless: 500,000 particles per minute
•Sitting with head, arms and body movement: 1,000,000 particles per minute
•Walking at 2 mph: 5,000,000 particles per minute
The study clearly illustrates that the removal of people from the manufacturing process is essential to minimizing risk to product sterility.
Advanced Aseptic Processing (AAP) is the utilization of automated technologies such as robotics and physical barriers in order to eliminate operator intervention with the process, open product containers, and exposed product contact surfaces. The key to AAP operation is maintaining absolute control of contamination sources through physical and aerodynamic means against contaminant migration into the sterile environment.
Isolators & Restricted Access Barrier Systems (RABS)
Isolators and Restricted Access Barrier Systems (RABS) are rigid wall mini-environments that provide a physical and aerodynamic barrier between the operator and the sterile drug manufacturing process enclosed within the interior environment. Both isolators and RABS, when operated properly, will provide an ISO 5 cleanroom environment meeting the regulatory requirements for particulate and microbiological concentrations. Although the internal requirements are the same for RABS and isolators, there are several key design features that differentiate the two systems.
An isolator is a totally enclosed and sealed stainless steel glove box t
DSC00881.JPG
ype system. The isolator has an air handling system that provides HEPA filtered air to the interior in a unidirectional down flow pattern. The air handling system can be designed to provide the isolator interior with positive or negative pressure. A positive pressure isolator is used to protect the interior environment from ingress of any contaminants from the background cleanroom. Negative pressure isolators are used for containment of biological or chemical products that are highly toxic and hazardous to the operator. Since the interior of the isolator is sealed off from the background cleanroom, operator access to the interior is done through glove ports or half suits. Sterile containers, stopper components, and environmental monitoring materials are brought in to and out of the isolator through air locks, mouse holes, and devices known as Rapid Transfer Ports (RTPs).
One of the major advantages that isolators have over RABS type systems is that the interior can be bio-decontaminated through a automated process commonly using hydrogen peroxide vapor (H2O2). This automated bio-decontamination process allows for a repeatable and consistent high-level bio-decontamination of the interior, thus the Sterility Assurance Level (SAL) is improved significantly over conventional cleanroom manufacturing.
Restricted Access Barrier Systems (RABS) Attributes
Restricted Access Barrier Systems (RABS) also offer a high level of product protection and contamination control. Unlike isolators they use a combination of physical and aerodynamic barriers to prevent ingress of contaminants into the interior environment. The physical barrier is similar to machine guarding having glass or polycarbonate doors with stainless steel walls that totally enclose the machinery with an air handler supplying HEPA filtered, unidirectional airflow providing an ISO 5 environment.
RABS operate with a positive pressure and a high air exchange rate relative to the background cleanroom. RABS are typically unsealed barriers having the HEPA filtered air supplied to the RABS interior and exhausted through a gap between the RABS walls and the equipment. RABS that exhaust to the background environment are referred to as open RABS.
An open active RABS has the air handler integrated into the barrier system. A passive open RABS is a barrier system that is built around equipment installed below air handlers in the background cleanroom, which provide the ISO 5 environment. Closed RABS offer another option and are by design sealed isolators that can be positive or negative pressure, but are manually cleaned and bio-decontaminated rather than utilizing an automated bio-decontamination process typical of isolators. Like isolators, introduction and exit of materials is done through mouse holes, Rapid Transfer Ports (RTPs) and pass throughs. Glove ports and half suits are also used to further separate an operator from the sterile interior of the RABS.
In the early implementation of the RABS concept by the pharmaceutical industry, many companies considered any enclosure around the process a RABS, claiming that it provided enhanced sterility protection over conventional cleanroom manufacturing. But in many cases operators would continually open the RABS doors to access areas within the critical zone to perform an intervention. Thus defeating the purpose of the RABS and creating an unpredictable level of contamination. Ultimately, RABS can meet the requirements of Advanced Aseptic Processing if it is used as intended; separate the operator from the process. RABS can only achieve this criterion when any and all open door interventions are prohibited.
Robotics in Aseptic Processing
Aseptic manufacturing in general is a highly repetitive activity that requires a high degree of reproducibility in order to create a high-quality product. Robots are the ideal platform to provide the highly accurate and repeatable operation demanded by aseptic processing. Additionally, robots can operate in environments where humans cannot. This becomes particularly important in applications that require containment of highly active and potent compounds. Robots also can be safely integrated into critical aseptic areas, because they generate extremely low non-viable and viable particulate levels having compatibility with ISO 5 environments.
To further advance the compatibility of robotics with isolators and advanced aseptic processing, robot manufacturers such as Stäubli Robotics has developed the TX series Stericlean and HE 6-axis robot arms, which are compatible with sanitizing using IPA (Isopropyl Alcohol) and bio-decontamination with sporicidal agents and vapor phase hydrogen peroxide (VPHP).
Isolated robotics in aseptic manufacturing has one particular advantage over traditional aseptic machinery; flexibility. Robots are completely adaptable and can change with the product or process. If the current application or the container format has changed, the robot system can be reprogrammed for another manufacturing process with minimal investment.
To adapt the robot to another application it would require new or modified end of a
IMG_7258.jpg
rm tooling, processing fixtures, programming, and possibly some additional utilities like vacuum and sterile air. The turnaround time and resources required to reconfigure a robot is considerably less than the investment in a new, dedicated machine or filling line.
Robot tool charger technology is widely used by other industrial manufacturing sectors to maximize the flexibility of robot systems, but remains untapped in pharmaceutical applications. Tool changers allow the robot to quickly couple and decouple the end of arm tooling to perform other manufacturing operations that cannot be performed on a single tool. It is conceivable that a single aseptic filling line could be designed for multiple container types (i.e. syringes, IV bags, vials, etc.) where the operator would only have to provide the proper tool to the robot for the particular container type, thereby allowing the same manufacturing line superior flexibility, with rapid changeover.
Robot Safety Equals Product Safety
In typical industrial applications a robot cell is enclosed with a safety fence having a combination of light curtains, laser area scanners, electronically interlocked doors, and awareness signaling to safeguard the operator from the "restricted operating space" of the robot. ANSI/RIA R15.06, Safety Requirements for Industrial Robots and Robot Systems, provides the designer and integrator of the robot system standard methods for assessing risk to operator safety, defines the requirements for protecting personnel interacting with or near the robot system, and assists with devising strategies to mitigate the level of assessed risk.
The combination of Isolator-Barrier technology with robot safety requirements ensures that protection of the critical zone is maintained during aseptic production. With isolator integrated robots, the isolator walls become the safety fence encircling the robot. Light curtains detect operator presence at the glove port(s), and the access door(s) are electrically interlocked, mechanically prohibiting the door(s) from being opened. The electrical outputs from these safety devices can be recorded and attached to the product batch record.
Additionally, quality control personnel can verify that the recorded interventions were validated per media fills and follow the process SOP's (Standard Operating Procedures). In a RABS application, the designer of the control system can utilize these safety devices to their advantage by developing a systematic approach through the machine control architecture to mitigate contamination risk during an open door intervention. This strategy would not allow interventions to take place or manufacturing to resume unless certain conditions are met.
This reduces contamination risks by having the control system walk the operator through a defined validated process, when an intervention is absolutely necessary. For example, the robot could be programmed to move the tool to the farthest point away and above the intervention location (near the supply HEPA filter for example) prior to the door being electronically unlocked, thus minimizing the contamination risk to the product contacting elements of the robot tool.
Summary
They key to Advanced Aseptic Processing is the elimination and absolute control of all sources of contaminants, most importantly human generated contamination. Robotics and isolator-barrier systems will be the core technologies that further this initiative. A properly integrated robot system that is compliant to ANSI/RIA safety requirements combined with a properly designed and implemented isolator-barrier system offers a flexible robotic cell that is compatible with the strictest of regulatory standards. Ultimately, the many layers of operator and product protection provided by isolated robotics offer superior control over ingress of contamination when compared to traditional cleanroom manufacturing and thereby protecting product quality and minimizing risk to patient safety.
About the Author(s)
You May Also Like


