January 29, 2014
 Immediately following the horrific events of 9/11, President Bush and the U.S. Congress set in motion a number of strategies to fortify the nation's defenses against terrorist acts, both conventional and unconventional. With the anthrax attacks, attention was sharply focused on the state of U.S. preparedness in the event of a biological assault, and in particular the country's ability to respond to a release by terrorists of the smallpox virus.
Immediately following the horrific events of 9/11, President Bush and the U.S. Congress set in motion a number of strategies to fortify the nation's defenses against terrorist acts, both conventional and unconventional. With the anthrax attacks, attention was sharply focused on the state of U.S. preparedness in the event of a biological assault, and in particular the country's ability to respond to a release by terrorists of the smallpox virus.
|
Precision chose four-side-seal packaging machines to pouch its smallpox needles at up to 750/min. |
|
Pouches exit the sealing machine in strips of 20 units, each of which contains five individually sealed needles. |
Smallpox, a highly infectious disease with a more than 30-percent case-fatality rate, is believed to have first appeared around 10,000 B.C. and, through the ages, has devastated populations globally. Until 1967, it is estimated that 2 million people/year died from the disease. While the first vaccine for smallpox was developed more than 200 years ago, it wasn't until the introduction of the bifurcated needle, a vaccine delivery system invented by Dr. Benjamin Rubin in 1965, that global vaccination was possible. Rubin's invention evolved from a sewing-machine needle with the top of the eyelet removed. The fork-like design allowed just the right amount of vaccine to be held between the tines for introduction into the patient's arm. In 1980, the World Health Organization declared that the virus had been eradicated, and routine vaccination against the disease was discontinued worldwide.
After 9/11, without a single person in the U.S. having been inoculated against smallpox for more than 20 years, the virus emerged as "a lethal bioweapon," according to the Centers for Disease Control and Prevention. To decrease America's vulnerability to such a bioterrorist threat, the government made a commitment to expand its National Pharmaceutical Stockpile to include enough of the smallpox vaccine to inoculate every one of the country's 288 million residents by the end of 2002. In October, 2001, Time magazine reported that the nation's existing supply was estimated to be at just 15 million doses. "And," the article continued, "there are plans afoot to purchase another 300 million."
In late 2001, when the U.S. government announced its intention to expand the National Pharmaceutical Stockpile with the purchase of 300 million units of the vaccine for the deadly smallpox virus by year-end 2002, no business could have been more stimulated–or more overwhelmed–than Denver, PA-based medical device manufacturer Precision Medical Products, Inc. The government's decision meant that Precision, the original manufacturer of the bifurcated needle and the only supplier of the product in the U.S. until 2002, faced a windfall of business from a formerly flat market. Says Bob Rhoads, vp, manufacturing and human resources for Precision, "It's a product that we'd been making for 40 years, but, once smallpox was eradicated, we did not have to manufacture it in any significant quantities for nearly twenty years."
The bad news was that equipping Precision's facility to produce the massive payload was no easy task, especially given the strenuous deadline. "We were under tremendous pressure," adds Rhoads. "Last year at this time, I think I had crisscrossed the country several times trying to find a source for the packaging equipment."
One year later, after the installation of three Doyen Medipharm four-side-seal packaging machines, equipped in an emergency installation with ink-jet printers from Nutec Systems, Rhoads says that Precision has delivered the goods in full.
Moving into high gear
Precision Medical Products, founded in late 1997 through a management buyout of Arrow Precision Products, Inc., has more than 50 years of experience in the development and assembly of medical components and devices for some of the world's leading pharmaceutical and medical device manufacturers. The company, which will be expanding into a 100,00-sq-ft facility later this year, presently operates a 38,000-sq-ft plant, and staffs 150 employees.
Packaging operations, while secondary to Precision's main business of medical device manufacturing, are crucial to its success and have been developed to match its customers' needs. Form/fill/seal, pouching, bag sealing and tray packaging are just a few of the processes used.
|
Two controllers are used on each pouching machine, with five printheads that print 10-across onto the bottom paper layer. |
In late 2001, plans were underway to enhance the company's bifurcated needle production and packaging capabilities on a smaller scale. For years, Precision had produced a very limited number of the needles for use in allergy skin testing, but a pending contract had them looking at solutions to help them "get back up and manufacturing several million of the needles," says Rhoads. "We were formulating all those plans when September 11 happened, and then everything changed," he recalls.
The sole supplier to U.S. pharmaceutical companies and agencies with the smallpox vaccine, Precision jumped into action, revising its plans from the purchase of new low-volume machines to a search for high-speed equipment.
New design ensures sterility
With reinvigoration of Precision's bifurcated needle packaging capabilities came the opportunity to redesign the needle packs for enhanced sterility. Previous packaging consisted of 100-unit packs, says Rhoads. "But," he explains, "if you opened the pack for just one needle, you would expose all 100 of them, and your sterility was compromised on the other ninety-nine." Thus, a new single-unit pack was created that allowed each needle to be individually sealed within a flexible film pouch.
Measuring approximately 4.531 in., the new packs are made from a clear, nylon-based top layer of film sealed to an opaque, latex-reinforced paper bottom layer, both supplied by TOLAS Health Care Packaging.
According to Precision project engineer Adam Doughty, the pouch's clear, top film was selected because it allows the user to see the needle inside and determine its orientation, and because of its puncture-resistance. The bottom paper layer, he explains, offers a medical-grade, permeable barrier that allows for ethylene oxide sterilization. While the porous substrate allows EtO gas to pass into the package, "bacteria and other bugs can't make it through the same membrane," he says. The paper layer was also chosen for its printability, which allows Precision to code the single-unit packs with product information and lot number.
Individually sealed needles are supplied in strips of five, with a peel-away end at one corner. Before they are sterilized, strips are packaged 20/carton, case-packed in quantities of 5,000 needles/case, and palletized.
Needle-feeding a challenge
In selecting the right equipment to package the needles, Precision had to take into account several unique aspects of the job. The needles, measuring about 2.5-in. long and approximately 0.038 in. dia, are not symmetrical, so they require a very special type of feeding system. Feeding such small items at high speeds so that they orient correctly "is probably the trickiest element of the job," according to Rhoads. "The needle has to be straight because you don't want it getting into the seal; that will compromise the seal's integrity," he explains. Needles also have to be oriented so that the blunt side is presented upon opening of the pouch, he adds.
Although Precision considered using form/fill/seal technology for the project, the best solution, they determined, was to use horizontal dwell and wrap equipment from Doyen Medipharm. Between January and May of 2002, three Doyen HDW four-side-seal machines with custom tooling and feeding systems were installed at Precision's plant. To make room for the new lines, the company moved some of its warehousing off-site, converting a 3,000-sq-ft area into a Class 100,000-certified cleanroom in a matter of weeks.
The feeding system for the machines was engineered by an outside firm, in cooperation with Doyen, and is based on specialized proprietary feeding systems already in use on some of Precision's other manufacturing lines. Doughty notes that the existing feeder design was enhanced with additional servo motors to increase the speed of the system. Hand-loaded into the feeding system, needles are fed 10 at a time across the 10-in.-wide paper rollstock at a speed of 750/min at the point where the top, film layer meets the bottom layer. Sensors detect missing needles and trigger the feeding system to make up the balance.
"The needles have to be straight, and they all have to line up," says Rhoads. "The Doyen machine is able to place the needles between the web, so that they are always held in position and can't roll."
After feeding, the pouch material with the needles sandwiched between the layers then advances to the heat-sealing unit. During heat-sealing, the system fuses the top and bottom pouch layers together around each needle. Features of the sealing-unit platen include continuous-motion, reciprocating action for increased throughput, as well as multizone control for easily validated control of all sealing parameters.
Once sealed, the webstock is slit and perforated lengthwise with a knife supplied by Mario Cotta, leaving two 5-in. webs, each five needles across. Before the webstock exits the machine, it is cut widthwise by equipment from Cut-All Tools into units measuring 4.5 x 5 in. that contain five individually sealed needles.
|
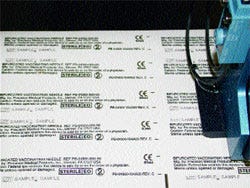
Text is printed in a 3/32-in.-high typeface, along with a bitmapped CE-Mark on the bottom paper layer of the pouchstock. |
Throughout feeding and sealing, a number of sensors, supplied by Allen-Bradley, Banner Engineering and others, evaluate pouches according to predetermined parameters, such as web splice, inspect for missing product, and monitor sealing temperature and pressure. Pouches not meeting the specified parameters are automatically rejected from the line, so that operators at the machine's tail end are always presented with 20 packets of fully sealed strips with five needles per strip.
According to Rhoads, job setup and changeover is minimal, as the bifurcated needle is the only product run on the line. The biggest part of the job, he says, is clearing the line of all needles and printed materials from the previous job, and then wiping down the machine with alcohol for sanitation purposes.
Ink-jet provides ideal solution
In May, 2002, with the three high-speed packaging machines installed, Precision began a race against time to fulfill its customer's immense order by the year-end deadline, until a "near-catastrophe" stopped the project in its tracks. A critical component of the job was the printing of product information and lot number on each single-needle pouch. However, the thermal-transfer printers originally installed on the Doyen machines to print the information on the paper web were "totally unreliable," says Rhoads, causing Precision to shut its operations down for several weeks.
Doughty recalls that when Precision approached other thermal-transfer printer suppliers about the project, "they wouldn't touch it." He notes, "It was very difficult to print on the paper and on the nylon, especially with the detail that we needed."
In June, 2002, Precision industrial engineer Barry Kalbach was sent to the Medical Device Manufacturers East trade show to find a solution. There, he discovered Nutec System's m600 ink-jet printer, which uses a single, disposable printhead/ink-supply system from Hewlett-Packard Specialty Printing Systems to print industrial applications at up to 600 dpi.
Within a week and a half, Nutec had come to Precision's rescue, providing the company with an off-line printing unit for testing. The printer allowed Precision to output 40,000 needles/day with extremely high print quality, relates Doughty, so an order was placed for six more units–two for each of the three Doyen HDW systems.
Installed at the front end of the packaging machines, the m600s feature five printheads apiece, each driven by two controllers, that are mounted above the paper rollstock. During operation, each printhead prints two sets of information onto the paper web below, allowing for 10-across printing. Text is printed in a 3/32-in.-high typeface, along with a bitmapped CE-Mark, at a speed that matches the output of the HDW.
In every aspect, Precision has found that the ink-jet printers provide a far better solution than the thermal-transfer technology with which they started the project. Says Rhoads: "When we purchased the Nutec printers, we just wanted a good print, fast. That was our goal, because time was of the essence for us, and we just absolutely had to do whatever it took to get back in operation and be reliable. These printers met that initial requirement. Then, as we went along, we realized that they were actually cheaper to work with in terms of the direct costs, they were easier to work with from a maintenance standpoint, and they were able to do so much more with much better quality."
Benefits abound
The m600 is a totally electronic machine that uses a removable Hewlett-Packard ink-jet cartridge similar to those used on home-office computer printers. When the cartridge is empty, it is simply removed, and a new one is snapped in, "which is much more convenient than having to change over the ribbons used in thermal-transfer printing," says Rhoads.
In terms of consumables costs, Precision realized a $200/day savings per line during its peak production period, versus the cost of thermal-transfer ribbons. Cartridges, which are changed in groups of five, last approximately three days, or 3 million prints, relates Doughty. In contrast, the thermal-transfer ribbons required constant replacement, with each ribbon needing to be changed roughly three times a day.
Reliability has been greatly enhanced with the m600, too. Says Rhoads, "Thermal-transfer ribbons can get creased or spotted or torn. And, you have to deal with the take-up reel and alignment."
And, although changing printing-job information is only a matter of inputting a new lot number, it is still a much quicker process with the m600. For each line, the lot number must be typed in twice, once for each controller, as opposed to the 10 times per machine previously necessary to set up a thermal-transfer job. "The old system was very susceptible to error," says Rhoads, "but we haven't had those types of problems with the Nutec system."
Lacking mechanical parts and using a water-based, rather than a solvent-based, ink system, the m600 also eliminates the need for spare parts and reduces maintenance requirements. Total printer upkeep involves wiping down each printhead approximately every four hours, says Rhoads.
Another unexpected benefit of the switch from thermal-transfer to ink-jet printing is the increased sealing versatility it affords. With the former technology, Precision could not heat-seal anywhere within the print area, or else the print would lift off from the heat. Ink-jet printing is not affected by the high sealing temperatures, which means that Precision is now able print anywhere on the package.
A task well done
In a press conference held Dec. 13, 2002, President Bush revealed that the U.S. had accomplished its goal of stockpiling enough of the smallpox vaccine to inoculate all of its citizens in the event of a bioterrorist attack. For this, Precision Medical Products can take great satisfaction. Says Rhoads, "A Herculean effort was made by everyone here to make it all happen." Their efforts, combined with the reliability of the company's new high-speed pouching and coding equipment, should help more than a few Americans rest easy tonight.
More information is available:
Pouching equipment: Doyen Medipharm, Inc., 863/683-6335. Circle No. 213.
Printers: Nutec Systems, Inc., 609/912-0145. Circle No. 214.
Film, paper: TOLAS Health Care Packaging, 215/322-7900. Circle No. 215.
Perforator: Mario Cotta America, Inc., 513-792.5510. Circle No. 216.
Knife: Cut-All Tools, Inc., 800/225-9970. Circle No. 217.
Sensors: Allen-Bradley Co., 414/382-2000. Circle No. 218.
Sensors: Banner Engineering Corp., 888/373-6767. Circle No. 219.
Printheads: Hewlett-Packard Specialty Printing Systems, 858/655-3524. Circle No. 220.
About the Author(s)
You May Also Like