May 23, 2017
Many of FDA’s Unique Device Identification requirements have already phased in. The labels and packages of Class III and Class II medical devices, for instance, must now bear a UDI.
“But UDI is not over,” says Ardi Batmanghelidj, President and CEO of Innovatum, a software and regulatory consulting company specializing in life science labeling and Unique Device Identification (UDI) compliance. “In contrast, it is just beginning.
“Although many medical device manufacturers have complied, on-going maintenance for previously submitted UDI data will soon be enforced,” he tells PMP News. “And the regulatory requirements of UDI variants by regulatory bodies around the world will continue to make global UDI compliance a challenge for quite some time.”
Batmanghelidj explains 5 common UDI challenges and potential solutions.
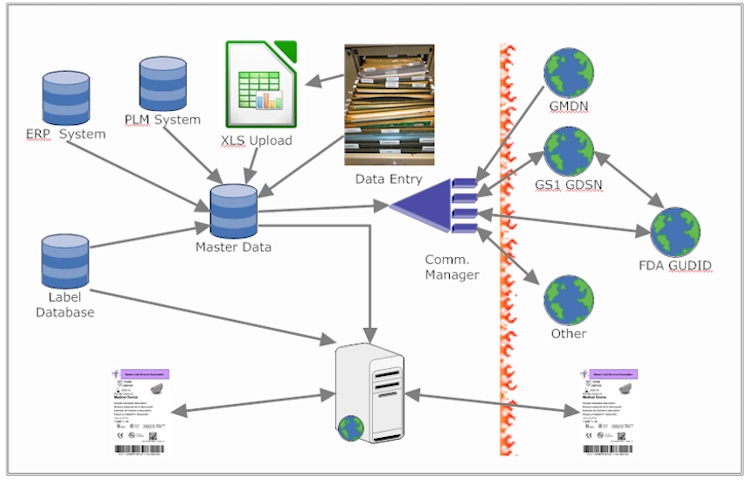
Communication. One of the challenges arises from miscommunication of labeling data. “Most medical device manufacturers inexplicably approach UDI by separating it into two different projects that are managed by two different departments,” Batmanghelidj says. “The labeling team is responsible for UDI data on the label, and the UDI team is responsible for UDI data management and submissions. Yet, most of the data overlaps and must be kept in sync! Think of a row in an Excel spreadsheet that contains fields with labeling specific data for an item. Now think of adding additional columns to house regulatory data within that same row. Since some of the data within that row is placed upon sticky labels and some of that very same data is also being submitted to regulatory bodies, this data can be stored once and reused. This ensures data quality. The problem of having to prevent a likely mismatch between the information that is submitted and the information used on the label is now gone. With an extension to what is traditionally considered to be enterprise labeling software, it is now possible to bring these capabilities to life.”
Using a common enterprise labeling software systems could promote better communication among packaging and labeling professionals. “Packaging and labeling is most often a symbiotic relationship. It is surprising how much cross training and mutual understanding of related business processes exists between these individual realms of expertise. But can software increase the synergies between these groups even further? Enterprise labeling software systems (ELS) provides incredible benefits to both groups by spanning cross functional needs with data that is common to both pursuits.”
The key is to “control the input and management of auditable data through a life sciences specific and adaptive master data management system (coalescing database),” he explains. “Life sciences specific extensible data management that is an integral part of an ELS allows this to happen. Each group gets a functional window into the approved data belonging to the other group. But that data is not limited to a small number of groups. It's reach and value can easily be expanded to other departments for other related pursuits. Packaging specifications, materials, sterilization approaches, etc. can be communicated across departments and corresponding label types and symbols can be assigned and managed utilizing online data access instead of Excel spreadsheets and emailed input forms.”
Integration. Integration can also be a challenge, so it requires careful consideration, Batmanghelidj says. “Not just the technical considerations that surround the way that each integration will be accomplished, e.g., push vs. pull, direct or through an intermediary, etc.,” he explains. “At a much higher level, one must consider the number of integrations that medical device manufacturers are being asked to make and at what point will it make sense to make just one integration. An integration that will allow all future communications needs to be satisfied from that one integration. Various supply chain partners subscribe to various data pools. Many of those data pools have unique communication requirements. Implementing and maintaining specific integrations to specific data pools to satisfy various supply chain partners are difficult and expensive to sustain. It makes more sense to get all data that needs to be communicated into a coalescing database first. Once the data is coalesced and available, plug-ins can be used to communicate according to the guidelines of various protocols. Flexibility is optimized, and the need to revalidate the entire system with the addition of a new supply chain partner is eliminated. Integration now becomes plug-in installation.”
Data Management. Data management can bring up issues, too. “The primary data management issue with UDI is deriving and maintaining a single source of truth," Batmanghelidj says. "Data validity and synchronization through reuse play a key role in ensuring that data being submitted and data being placed on the label match each other. These factors have much bigger implications when looking at the bigger picture.”
And “without a coalescing database, UDI will continue to create the greatest challenges for data management that medical device manufacturers have ever encountered,” he believes. “Excel has sufficed in getting companies to a starting point of compliance. The real challenge now is data maintenance and sustainability through reuse as other regulatory bodies around the world begin to announce their variations on the U.S. FDA UDI approach.”
Location. Many of today’s medical device companies have more than one location or entity involved in supporting UDI, potentially in more than one country. Multiple players can compound the aforementioned challenges. But strides are being made in helping medical device companies address destination labeling, localization, and globalization challenges, Batmanghelidj says. “Company divisions, contractors, suppliers, etc. can safely and effectively manage their own data through a browser, and this idea is truly revolutionary,” he says. “Built-in task dependency management and reporting, as well as granular auditability for changes to data, make the ‘manage the data that you are responsible for approach’ practical. Beyond the input and upkeep of data, great strides have been made in the ability to exploit the power of data in storage through interesting new associations. Versions of electronically stored documents can be associated with versions of item information and changes can be automatically reflected in the electronic posting of documents such as electronic instructions for use (eIFUs).”
Security. Thankfully, security doesn’t seem to be a huge issue for UDI, according to Batmanghelidj. “To date, security issues brought about by UDI have largely been a product of perception. A copious number of responses to initial drafts of the UDI rule ensured that information that could be used to a competitor’s advantage has been kept off the list of GUDID product attribute submission requirements. What remains is the level of confidence that medical device manufacturers have that data pool providers will maintain the confidentiality of their production data. Some companies go as far as to not trust data pool providers to submit to the GUDID and also share related production information with their supply chain partners. These medical device manufacturers have opted to set up separate threads of communications.”
Innovatum offers the ROBAR family of products to help with label design, management, and printing; regulatory data management and submissions; and eIFUs. “A comprehensive approach to labeling necessarily involves the sharing of data for the management of closely related pursuits,” Batmanghelidj says. “ROBAR is a robust enterprise labeling software system that includes a tightly integrated series of modules. These modules can be used in unison to manage the full labeling life cycle using shared data.”
ROBAR also includes a coalescing database, “and this opens a world of possibilities,” Batmanghelidj adds. “With an emphasis on a coalescing database at its core, the ROBAR family of products includes column level controls for data so departments, contracts manufacturers, and subsidiaries can fill in and maintain their own data. This capability streamlines and synchronizes both labeling and regulatory data. Label design and print integrates industry leading BarTender technology yet controls it in a 21 CFR Part 11 (title 21 of code of federal regulations) compliant way. Automated approval workflow and electronic submissions are made available at the click of a button. And eIFU management and posting is automated with information, which is correlated and synchronized from the coalescing database.”
For more details, visit www.innovatum.com.
Don't miss the 2017 MD&M East Keynote Speaker: Steve "Woz" Wozniak Co-Founder of Apple and Chief Scientist at Primary Data. He'll be speaking June 13, 2017, at 12:00 p.m. at New York City's Javits Center.
About the Author(s)
You May Also Like




