July 26, 2017
“Even though FDA’s enforcement of the Drug Supply Chain Security Act (DSCSA) has been pushed back a year, the illicit trade won’t stop,” warns Bob Miglani, speaking of pharmaceutical counterfeiting, diversion, and other criminal activity. Miglani spent 23 years working for Pfizer, and a year ago he joined Applied DNA Sciences to serve as chief of business development.
The firm’s DNA markers have already made “tremendous progress” in several pharma labeling and packaging pilots, he says, and the company is now working to address industry’s challenges—and perhaps reluctance—with employing a routine product authentication strategy.
“DSCSA is a tide that lifts all boats,” he explains, but he adds that “regardless of serialization,” there are still threats. “Serialization is digital, and anything digital can be copied and hacked. Threats and challenges will always be there for anything digital,” he says. “Companies need to refresh their toolbox.”
Layers of anticounterfeiting technologies can help counter these risks, just as they do in currency, he says. “Our technology fits into those layers,” he says.
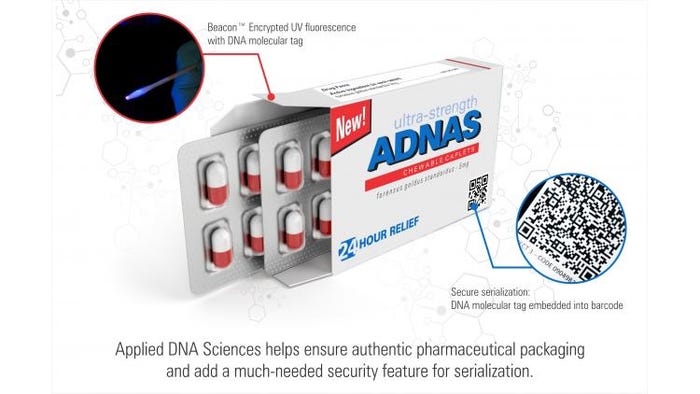
Applied DNA Sciences’s DNA markers—which Miglani describes as “molecular bar codes”—can be incorporated in “virtually any material.” They have been tested and validated in inks, varnishes, even embedded in materials, and the company has recently completed feasibility studies of tablet coatings. “With a significant proportion of the world’s tablets having a coating,” he says, “it is a seamless way to integrate our technology.”
The technology is also a robust one. The DNA markers have been employed in cash boxes that have an electronic system that sprays the cash with dye if breached. A thief may be able to wash out the dye, but not the DNA, so the crime can be linked to the person, he says. “We’ve already had 114 convictions in the United Kingdom,” he says.
And field DNA readers have gotten better, with faster readers and field-deployable authentication kits that can be carried onto planes, he says.
However, the challenge with authentication technologies hasn’t necessarily been the speed, but rather the frequency, he explains. “When the police bust bad guys in Poland, for instance, they look at the packaging and then call the drug company to verify. The security contact is located and sent the suspect product. Folks in the lab then test it and determine whether it is counterfeit. The company then needs to file a suspect product investigation report with FDA. All of this is a very reactive way of testing suspect products, and it happens a few times a year,” he explains. “It is useful, but not good enough.”
Pharma companies may hesitate to employ forensic authentication because of the effort behind investigation. Miglani says companies struggle with training employees, developing SOPs, access to systems, and accuracy.
But given the ongoing risks—and the fact that FDA is putting pressure on companies to monitor markets—“companies need to be more proactive and routinely audit and test products in the supply chain,” he says.
To address these challenges, Applied DNA is developing a new “authentication as a service” approach. “No longer do companies have to worry about testing,” he says. “We will do so in hotspots where there could be problems. We could provide each customer with an inspection officer who can test products in the field. We’ll produce quarterly audit reports and produce ‘heat maps’ showing, for instance, that 70% of the samples from one market are authentic and 30% are not.”
Applied DNA has performed such frequent testing in the cotton and tech industries. The company runs a full-service ISO 17025 accredited lab in Stony Brook, and its lab workers are trained like crime scene investigators, he says. “Our forensic reports have held up in court,” he adds.
The approach offers the potential for “a lot of data mining,” he says. “You can utilize analytics to shift strategies. If you see problems in one country, for instance, you can take a look at your distributors.”
Such data can also be used as a value proposition, he adds. “If you want more business from a hospital chain, you could show reports on testing of the supply chain. We are living in a world of total transparency. It is important to put all your cards on the table.”
Miglani advises pharma companies to look upon routine field authentication as just another means of auditing. “The pharmaceutical supply chain is used to auditing,” he says. “Pharma is very good at compliance.” And some companies would rather be given a certificate showing they have passed such audits, he adds.
He adds that Applied DNA has “learned that the approach must be seamless and integrated into the pharma supply chain ecosystem. The vastness of that ecosystem is massive in scale and complexity,” he says. “There is a lot of potential for our technology—pharma needs to reduce risk and differentiate its products, and FDA is watching pharma companies closely. For certainty in the marketplace, our approach offers a whole system to tag, test, and trust.”
For more details, see our 2016 article, "Turning to nature to fight counterfeiting."
**************************************************
MinnPack 2017 (Nov. 8-9; Minneapolis) celebrates its 15th year to bring you the latest developments in all things packaging as part of a comprehensive advanced manufacturing event. Sign up today to attend!
About the Author(s)
You May Also Like




