May 10, 2017
Given autoinjectors’ potential to ease drug delivery, interest and adoption in these devices are steadily growing. Research and Markets predicts a growth rate of nearly 19% for the global autoinjectors market during the forecasted period 2016-2022.
For these devices to truly have an impact, their performance must be thoroughly tested by device manufacturers, as well as the drug companies that adopt them. “Testing in conformance to ISO 11608-5 represents a critical step for manufacturers,” Lorraine McLean, Medical Industry Manager at Zwick USA, tells PMP News. “Some companies manage the manufacture of autoinjectors in house, while others turn to specialized contract manufacturing organizations. In both scenarios, testing plays a vital role in supporting product quality and safety. A variety of tests may be performed to support manufacturer protocols and to satisfy industry regulations. Contract manufacturing organizations handle a wide range of product designs and therefore seek platforms that offer flexibility and versatility.”
McLean says through her team’s discussions with these companies, a common market requirement has emerged—the need for a single testing platform that can test all relevant functions of an autoinjector before production batches enter the market.
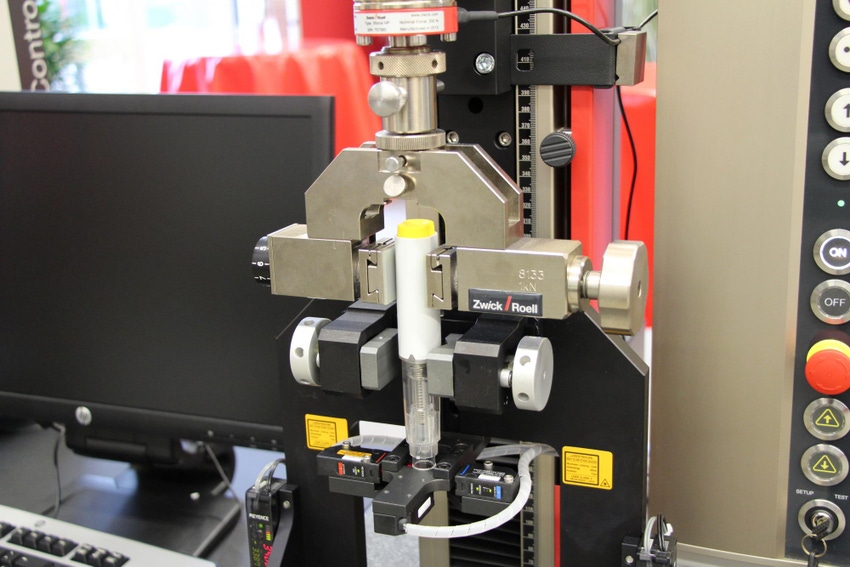
“Growth in market demand is placing greater emphasis on throughput,” McLean explains. “This phenomenon has motivated manufacturers to seek solutions that enable the full range of functional tests. Our automated testing system performs safety cap removal, determines the needle shield force, measures the activation force of the trigger button, and determines the error analysis of the needle tip with an HD camera. Our system also measures the ejection time, determines the ejected needle length, incorporates click detection during injection via a microphone, and automatically detects the fluid container and the maximum fluid volume."
The need to test these eight attributes “on an almost continuous basis” inspired Zwick to develop an autoinjector testing system based on its dual-column AllroundLine materials testing machine. “The system is able to perform all of the required tests on a single test specimen in one single test run, reducing the number of specimens required and increasing throughput,” she explains. “The machine offers different levels of automation to accommodate increases in specimen throughput—from a manual solution to a fully automated robotic testing system. A typical market solution is a semi-automated testing machine that involves an operator loading the test specimen, closing the safety door, and starting the test. From that point forward, all steps in the test sequence are carried out automatically by the machine within approximately two minutes per injector.”
Zwick’s machine employs a non-contact sensor that measures the injection time and the effective needle length by means of laser sensors. An integrated scale measures the quantity of the administered therapeutic, and an optional audio system may be added to detect the clicking sound that signals the beginning and the end of the injection, the company reports. Manufacturers may also add a high-definition camera to capture liquid emission. The recorded sequences can then be stored with the test results, fulfilling traceability requirements.
“Our testXpert III software controls the entire test sequence and is ideal for applications subject to industry regulation. The Expanded Traceability function enables lab managers to easily determine who was logged on, what tests were conducted, and what data was generated. Electronic data logging as well as electronic signatures are easily set up according to our customers’ individual requirements. They find this capability particularly useful in maintaining compliance with FDA 21 CFR Part 11,” says McLean.
Lab managers can assign staff member roles and administrator capabilities within the testXpert III software, and define user access levels according to internal training programs and protocols. “This capability is ideal for enterprises managing global programs that must fulfill requirements to ensure consistency and to validate manufacturing processes at multiple sites,” she says.
Further information on autoinjector testing is available on Zwick's website.
Click here to view a video on autoinjector testing on the ZwickRoellTV YouTube channel.
Zwick will be exhibiting in Booth #3233 at the upcoming CPhI North America in Philadelphia May 16-18.
Zwick USA is the North America subsidiary of Zwick Roell, a global manufacturer of material and component testing systems. Zwick USA addresses customer needs in the United States and Canada, the company reports.
"Global Auto-Injectors Market Insights, Opportunity Analysis, Market Shares and Forecast, 2017-2023" is report ID# 3940065 and is available from Research and Markets here.
About the Author(s)
You May Also Like




