December 1, 2015
As self-treating consumers reached for more product in the over-the-counter aisle, manufacturers rethought healthcare packaging for competitive advantage.
Pharmaceutical companies’ strategic goals and the trend toward consumer self-medication have been the predominate influences driving an emphasis on over-the-counter (OTC) and innovative packaging.
As patients have taken more active roles in their healthcare, drug companies used packaging to provide them with the information they sought to find. Marketers grasped the opportunity to sway the point-of-purchase decisions many consumers make while scanning the shelves of OTC aisles.
If sales were to be captured in crowded product categories, packaging needed to incorporate appealing graphics and clearly convey treatments’ benefits. Marketers sought differentiation with packaging and containers that were easier-to-use, and built brand loyalty with promotional tie-ins.
Product life cycle strategies encompassed Rx-to-OTC switches to tap into a new venue for sales, and often to counter lost revenue from a prescription drug losing its patent protection.
Drug companies found they had to fine-tune or in some cases completely overhaul a line’s packaging to be competitively viable in a category. Fresh ideas for graphics and containers appeared with new OTC launches as marketers addressed consumers whose first real glimpse of the product often occurred in a store visit.
When Walmart some years ago reported how consumers had embraced the “consumerized packaging” it has promoted for prescription drugs, it struck a chord with OTC marketers. Prilosec was sold in a seven-count blister to reinforce daily usage, and packaging for Claritin and private-label Loratadine debuted in seven-day formats, providing a valued-added dimension to the packaging.
Suppliers responded to the urgency for new packaging ideas with new formats, different materials, and advanced printing technologies. Converters took costs out of carton and label supplies, as clients sought to conform with a growing body of OTC labeling mandates from FDA.
“As recently as five years ago, there was not much emphasis on graphics for the label or carton,” Lance Vanden Brook, then sales and marketing manager, New Jersey Packaging, would observe in 1998. “With the mounting competition in the OTC market, we have seen companies change to much higher-end graphics. Drug manufacturers are looking for more unique packaging.”
National Label would invest in combination platform-style presses to better serve clients in the market for high-end results, Neil Sellars, then director of product development and marketing, National Label said in 2001.
“The packaging managers for OTC products are asking for graphic effects similar to those found on personal care products—everything from matte and gloss varnishes to screen effects that have helped personal care products stand out on the shelf. We are finding that variable repeat offset stations can produce the soft vignettes that really make a brand stand out,” said Sellars.
In 2010, Catalent Pharma Solutions identified OTCs as a high-growth-potential segment. “We are seeing the branded drug side declining as top-selling brands lose exclusivity and project modest growth for generics. The largest opportunity is in OTCs where Rx-to-OTC switches will be driving a high compounded annual growth rate. While plastic bottles are still by far the largest format for packaging of solid doses, companies are rethinking their strategies for improving patient treatment using blisters and more sophisticated cartoning with alternative carton materials,” said John LaHaye, then VP of business development, commercial packaging, Catalent Pharma Solutions.
The Drug Facts Label
To help consumers with their OTC product choices, FDA in 1999 issued the “Drug Facts” label requirement. The rule made label information more uniform and easier to read and understand with a Drug Facts box for information on the drug’s intended uses, active ingredients, directions for use. and warnings.
The rule prompted many manufacturers to rethink OTC packaging to meet compliance deadlines based on product approval dates and annual sales that stretched well into the next decade. By 2002 most of the 100,000-plus OTC drugs affected had to come into compliance.
“The biggest challenge will be to make sure you have appealing graphics, but you [must also] cover all the regulatory aspects, such as the six-point type size,” said Kumar Nanavati, director of packaging development, Whitehall-Robins Healthcare, the division of American Home Products Corp.
FDA recommended that the Drug Facts format be placed on both primary and secondary packaging but only required that it be visible to the consumer at the point of purchase.
Some sacrificed logos and graphics to avoid extensive package or label changes, taking advantage of scaled down type size and spacing requirements FDA allowed for packages with less surface area.
“Most times the issue can be resolved with the inclusion of a panel on the adhesive side of the label,” said Des Laffan, general manager of Pharmalabel said in 2001. “This does not cost as much as multi-panel or multi-ply labels. In many cases, reasonably priced solutions can be run without heavy make-ready requirements or special equipment.”
For others, the ruling spurred a wholesale reevaluation to liven up the packaging and reinforce brand identity.
“We are seeing a dramatic increase in the redesign of OTC packaging as a result of the FDA rule on labeling OTC products. Once the package concept is open to redesign, customers ask for graphics in combination with other package features like anti-counterfeiting features or enhanced child-resistant, senior-friendly closures,” said Dick DeWiggins of Mebane Packaging, Westvaco in 2001.
The pharmaceutical industry to a large extent pioneered safety and security features with OTC packaging, embracing tamper evident components and features to thwart counterfeiters. Keller-Crescent deployed microprint and wave rule features as low cost security features for OTC cartons.
In child resistant designs, Sharp Corp. offered pouches for OTC sample packs opened by folding the sealed foil at one corner to expose a notch for tearing.
As companies adopted extended content labels (ECLs) and cartons with extra panels for featuring more information, they sought shelf appeal with flashier graphics. Some companies saw an advantage in adding a folding carton. Others dropped the secondary packaging favoring wrap around or booklet styles labels affixed to the primary containers.
Regulation would continue to spur package reevaluation. In 2004, FDA required warning labeling for thousands of OTC drugs containing calcium, magnesium, and potassium. A few years later, OTC makers began to add panels to bottle and pouch labels to create room for warnings required on OTC pain relief medications.
While regulation over the years forced package changes, many otherwise jumped at the chance to render packaging livelier, more helpful to patients, and more efficient for retailers.
New packaging
VasoActive Pharmaceuticals used foil stamping and embossing in creating new brand identities for three flagship topical lotions in 2006. The design for Osteon and A-R Extreme lotions for muscle, joint, and skeletal pain, and the athlete’s foot remedy Termin-8 featured action themes to convey the attributes of the company’s proprietary PentoCore technology. A “V” logo and a reverberating wave graphic tied the family of products together, with differentiation between them rendered by graphic elements and color coding. The design helped the company move distribution from Web sales to the shelves of national retail chains.
Web site support was a central component to the launch in 2007 by GlaxoSmithKline of alli (orlistat), the first FDA-approved OTC weight loss medicine. A clean-looking and colorful starter kit featured seven booklets to help dieters learn about weight loss management and track their progress. Consumers enrolled for personalized emails, set up custom action plans, and accessed information on meal planning and managing hunger at myalli.com.
In a redesign of the packaging system for Bayer Aspirin and Aleve launched at retail in 2009, Bayer Healthcare created new user-friendly bottles and launched a first for Bayer and the analgesics category by eliminating the carton. Carrying over the brands’ traditional colors, the bottles featured new oval shapes with raised logo grips on the sides, and “soft touch” thermoplastic elastomer-covered caps.
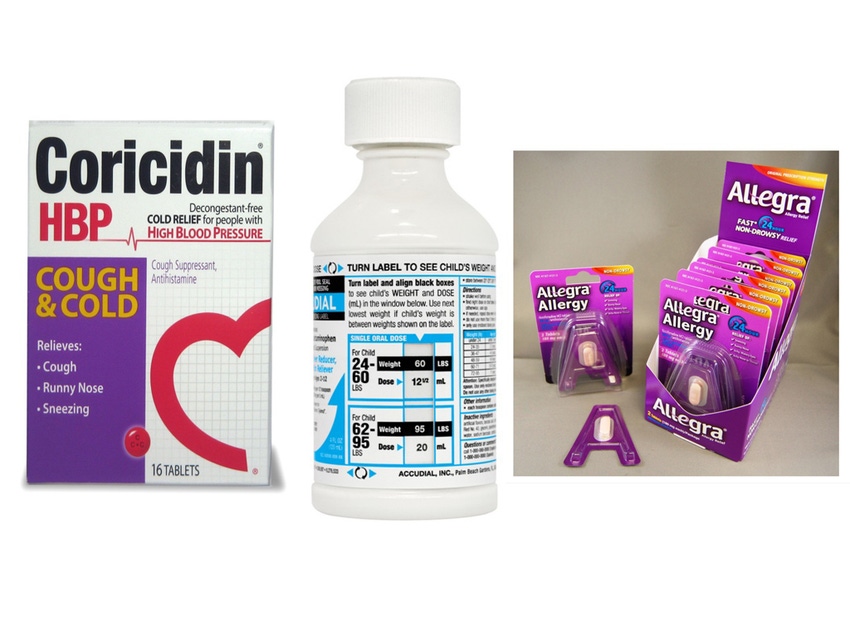
Shelf presence is a predominate concern in the cough-and-cold category where cold and flu sufferers are making quick decisions from among a host of multi-symptom, multi-ingredient products. Aiming to boost shelf facings and sales, Schering-Plough in 2010 revised packaging for Coricidin cold and flu products to highlight a key point of differentiation with other leading brands—the absence of decongestants that have been linked to high blood pressure. The four-sku line featured a red heart icon with an “HBP” (high blood pressure) designation to impart emphasis on the HBP benefit.
Schering-Plough sought to capture more facings on store shelves with a redesign of packaging for Coricidin cold and flu products that referenced a key point of differentiation; a decongestant-free formulation beneficial for people with high blood pressure.
Pfizer Consumer Healthcare in 2010 launched Advil Congestion Relief with an eye-grabbing color scheme. For the first Advil product containing ibuprofen and the nasal decongestant phenylephrine, and the first Advil congestion relief product available off-the-shelf, Pfizer used a carton-affixed extended content label to clearly communicate the ingredients and indications.
3M Healthcare in 2010 replaced staid and confusing packaging of the seven-product Cavilon Professional Skin Care line in favor of a simple, intuitive design with bold colors and upsized fonts.
“As a leader in package innovation and design, we want to make sure we are consistently and constantly driving loyalty with packaging that promotes ease-of-use and quality of care. “We asked, ‘How do packaging and package design meet user perceptions, preferences, and needs, so that every corner and element of the package has a very specific reason and purpose,” said Brian Ksicinski, Cavilon brand marketing communications.
In revamped packaging for the OTC probiotic supplement Align, Procter & Gamble featured a Daily Digestive Tracker and Guide to help patient track their progress with a treatment that naturally balances the digestive system.
When Allegra went over-the-counter in 2011, Chattem, Inc., the U.S. Consumer Healthcare Division of sanofi-aventis U.S. developed the packaging for skus in 12 different sizes, in blisters, bottles, and carton formats. As colors and design elements from the existing brand were carried over to maintain brand continuity, retail bottles feature child-resistant, squeeze-and-turn closures with no bottle neck or shoulder exposed to render a sleek seamless look, with pull- tab induction seals for easy opening.
Citing pediatrician support for syringe dosing as more accurate, safe, and hygienic, Perrigo Company in 2011 changed the dosing device for OTC pediatric suspensions from a dropper to a new syringe dosing system designed to help consumers more clearly dispense the proper amount of medication.
In launching DolipraneLib, an OTC version of Doliprane (paracetamol) in 2011 into French pharmacies, sanofi-aventis saw in the Burgopack a modern design that would differentiate the product in the crowded analgesic category. Consumers valuing portability could benefit from the package’s novel sliding mechanism that keeps product and information easily accessible.
Labeling
As converters offered a host of new label styles and insert formats, marketers favored the solutions to fit in more copy, bill-board enhanced graphical elements, and help promote compliant dosing. ECLs-- supplied in wrap around designs and in fold out or booklet formats—provided real estate for couponing and cross promoting.
“Manufacturers want more copy, bolder copy, and more colors, and it is up to us to respond,” said Les Schriber, then regional vice president, pharmaceutical division, CCL Label, Inc. in 1998. CCL Label offered its two-ply Spinformation label with top and bottom layers providing 75% more space for graphics.
Bill Mitchell, president of the Printing Components division of contract packager and printer PCI Services, a division of Cardinal Health in 2001 cited demand for multiple-color labels with hot-stamped graphics noting ”the days of black type and one color are rapidly disappearing.”
The Nosco Printing Co. would supply the Fix-a-Form multi-panel label--for bottles or cartons-- as a vehicle for shelf presence, communicating product benefits, and adding multiple languages and security features.
“Multi-panel labels are in more demand due to minimum point-size requirements and offer the potential to eliminate inserts in some challenging applications. It becomes an interactive piece and offers a manufacturer the chance to better communicate with the public.” said Nosco’s Ed Hudson, executive vp said in 2004.
In discarding carton packaging for its contact lens solution, Allergan in 2009 opted for a full-body shrink sleeve on a new hour-glass-shaped Rocket Bottle. Besides serving as a label solution that adhered to the bottle’s curves, the sleeve offered distinctive shelf appeal.
For accuracy in children’s dosing of cold and allergy OTCs, the Children’s AccuDial line featured a patented rotating label that shows parents the proper dose amount based on a child’s weight.
And companies leveraged inserts for cost efficiently fitting in information and graphics in some cases with full-color photos and friendly font sizes to guide user dosing. Tom Flottmann, president of The Flottman Co. saw more colorful insert graphics being used to ensure proper product usage and support marketing objectives in 2001.
“OTC products such as cough syrups offer manufacturers tremendous cross-product promotional opportunities. Using a graphically appealing insert the marketer can encourage the consumer to use another product,” Flottmann said.
As inserts ballooned in size, converters upgraded their inset folding machines from Vijuk Equipment, Inc. Apex Graphics in 2011 added a Vijuk model that increased flat size by 20% for sheets folded into 210 panels.
“Customers are open to just about anything you can present if it can reduce their costs. They want to know how they can get their costs down, and how can they can keep reducing them,” said Apex Graphics managing director Stephanie Magill.
Cartoning
Companies with well-established brands tend to scale back on the packaging to maximize profits. But for new product launches or reviving an old product, cartons offered shelf clout. Carton manufacturers added extra panels for handling more patient text. Using a carton, a packager could use a more manageable and lower-cost, standard leaflet fold size as an alternative to tightly wadded inserts and outserts. Companies with high-value OTCs might have electronic article surveillance tags or other security features embedded.
“We are seeing unique carton designs that almost resemble the cosmetic world, such as with chisel tops. Gold stamping and additional colors on the carton are a more common request,” said Joe Elpick, president, of Colonial Carton Co. (since rebranded as 3C Packaging!) in 1998.
Foil stamping was used to make product more recognizable and convey an image of quality, along with brighter and more vibrant colors, and holographic accents. Brighter UV coatings caught and reflected store lighting.
“The consumer reacts to a package in a sixth of a second so you have to grab the eye that quickly. Customers want higher-gloss coatings, embossing, foil stamping, and other enhanced graphic requirements, especially in OTC packaging which is so competitive,” said Tony Petrelli, vp marketing and business development, Caraustar Custom Packaging Group in 2000.
Carton Service Inc.-Packaging Insights (since rebranded as Pharma Packaging Solutions) in 2006 used a high-speed windowing machine to create a see through feature in OTC packaging designed for Merz Pharmaceutical’s Appearex nail treatment.
“Customers recognize the bill board advantage cartons provide in OTC packaging and they don’t want to give up shelf space. They are often in the market for metalized and UV inks, and raised impressions to make packaging pop out on the shelf,” said Paul Glintenkamp, then director of pharmaceutical packaging at Carton Service.
Meadwestvaco’s packaging for the Kinerase skin care regimen featured a cartoned thermoformed tray with fifth and sixth carton panels to convey education and scientific information on the product’s efficacy.
Converters invested in technology to supply cartons with leaflets pre-integrated. The solution reduced SKUs and manufacturing process steps at the packager, while keeping the literature readily accessible for the consumer. Packagers increased line speeds and production efficiency in eliminating insert insertion jam ups using the format.
The integrated carton/insert solutions would be offered by converters including Chesapeake Pharmaceutical and Healthcare Packaging, Nosco, Keller Crescent, Pharma Packaging Solutions, Cortegra,, and 3C Packaging! Fleet Laboratories adopted the InfoSert from Pharmagraphics (since acquired by the Clondalkin Group) with insert pre-glued to the inside of the carton.
And technology helped manufacturers get product to market faster. For the initial stages in carton and label design, electronic pre-press ensured graphics are compatible with the process selected and the capabilities of their supplier. With computer graphic design designers could see what worked in the carton manufacturing before it went into production.
Eight color presses enabled more graphics complexity, while digital printing supported cost effective production of smaller runs. John Henry would configure its business to handle short-run color to meet the needs of private-label products with Indigo, Xeikon, and Heidelberg equipment presses.
“Private-label OTC applications typically demand smaller runs than national brands but they are still treated like brands by the major retailers. There is a tremendous amount of time, effort, and money going into making OTC private-label products stand out on the shelf,” said Mike Longo, vice president of sales and marketing, John Henry.
Containers
Brand personalities were recharged with new style containers such as the Mega Pumps’ Airless Dispenser from Mega Pumps, a system that protects product from exposure to the air while additionally enabling precise topical dosing. Dr. Scholl’s deployed the container for its regeneration cream, and Sixtus for personal care products. Bayer packaged its Bepanthen Scar Gel in a Mega Pump system with integrated massage ball.
Designs like the Duma Pocket from Superfos Pharma Pack emphasized portability. The oval shaped container features a flat facing for easy label placement and a literature compartment. The Duma Pocket is designed to comfortably fit in a clothes pocket or slip through a mail slot after an Internet sale.
Meadwestvaco in 2009 developed the Med-Easy package concept as a portable, booklet-style package. Leaflets and blisters are held in vacuum forms to keep the components with the package as the patient uses one hand to click the blister out of the pocket and dispense the pill. A merchandising system featured trays and pillars supporting convenient pack-out and in-store presence. “With Med-Easy, we have developed a complete solution that considers how the packaging will work in the supply chain and in the stores. We can get more product on a pallet and more on the shelf,” said David Spackman, then director of healthcare packaging (Europe), MWV.
Packaging addressed concerns over pediatric dosing. Comar, Inc. made its AccuCup available as a stock design. The dosage cup features measured markings printed with food grade inks. AccuDial Pharmaceutical, Inc. launched a line of pediatric products in a weight-based dosing system designed to prevent under- and over-dosing. The system featured a bottle label from CCL Label in which the user rotates a top label to display the child’s weight, and see dosing information revealed on an inner label.
Chattem developed novel trial packaging for Allegra OTC tablets designed to appeal to on-the-go lifestyles and expand distribution to consumer-convenient locations. Tablets packaged in “A” shaped blisters are held in a clam shell that breaks away from the card to become a reusable, open-and-snap-close “nomad” package.
Materials
Materials helped with cost reduction, as customers looked to consolidate material choices for product portfolios.
Flexcon designed films to be used for all the products in a particular brand family. “A drug manufacturer will use the same film for its cold remedy, its flu remedy, and its expectorant product. The specifications for each label are the same but with different printing and graphics,” said Rick Harris, then manager, packaging sales and marketing Flexcon 2001
“The push for cost reduction in both ethical and OTC is very real. The biggest change I see in specification and production of labels is the drive to standardize materials. Most drug makers feel that if they limit their choices of label materials, they can streamline the entire specification process,” said PCI’s Bill Mitchell that year.
Flexcon was among suppliers providing materials that could flawlessly accept color. “Using a film that is surface treated and top coated for print receptivity for multiple colors is a must. We have seen a growing demand for polyethylene and polypropylene film label materials for OTC applications. Printers are investing in equipment to run seven and eight colors for these OTC applications. And customers are combining color with clear, matte, and white films,” said Flexcon’s Harris.
Label Express Inc. worked with Avery Dennison to create a functional, lower-cost solution for external content labeling needs. Avery supplied a polyolefin film called Fasson Primax that served as the base label stock material for Label Express’s Reveal Estate labeling technology, which uses the back of the label to expand label space by 66% or more.
Alcoa Packaging launched a “consumer-friendly” peel-push lidding for blister packaging that used a 50-µm PET film instead of paper. The Safety-Pak Plus lid stock addressed ease of use and child resistance in offering enhanced peelability and better puncture resistance. An acrylic print primer coating enhanced printability/
In 2009, Ampac Flexibles featured its Flexi-Free CR barrier overwrap film laminations for OTC applications, providing tear and puncture properties for child resistance while enabling ease-of opening by seniors. The film was first used as an overwrap for a plastic vial-based delivery system for an OTC allergy medication.
Promotions
If new packaging started a conversation with the patient, promotional campaigns—with tie-ins to retailer, media advertising, and web-based resources helped complete the message.
In-store merchandising and promotion is a leading tactic for lending a second wind to an established brand.
When Walmart was looking for a game-changing solution in a smoking cessation program, GlaxoSmithKline Consumer Healthcare in 2011 developed a multi-dimensional campaign for Nicorette and other GSK nicotine replacement products. As product was featured around the store, shelf displays advertised the availability of best practice ways to quit and purchasing consumers were offered rebates for enrolling in a professionally-guided behavioral support program.
“The previous category card was entirely product focused, advertising only the choices of patch, gum, or lozenge. We transitioned from a product-oriented category to a quit-oriented approach, which was pretty remarkable for a retailer,” said Janna Lacatell, solution design manager, Healthways.
In 2010, Catalent Pharma Solutions moved OTC healthcare packaging into the digital age with the debut of its mobile marketing solution, Media Enhanced Packaging. Industry began to see the opportunity to use “smart packaging’ to engage an exponentially growing base of smart phone owners.
As an exclusive partner with Digimarc for healthcare printed packaging in North America, Catalent applies Digimarc digital water marks imperceptible to the human eye into existing art work for printing labels, cartons, or other printed components. Product manufacturers can direct owners of web-enabled smart phones to scan the package and connect with all manner of Internet-based instructions, offers, rich media content, and even communication with caregivers.
“Brand owners can influence or persuade the consumer while re-enforcing the brand. One big benefit is there is no additional real estate on the package so you are driving out packaging costs,” said Victor Dixon, then vp and general manager, Catalent Pharma Solutions.
Sustainability
Momentum for sustainable packaging was boosted by Walmart’s release of its Packaging Scorecard in 2006. Diamond Packaging the following year would launch the Diamond Greenbox program promoting “green” practices in design, material selection, at the plant level, and in production.
“Wal-Mart’s Packaging Scorecard tool has helped put the concept of sustainable packaging in the forefront of many companies’ and consumers’ minds and thus has been a key driver [for companies] trying to understand what sustainability truly is,” commented Dennis Bacchetta then director of marketing, Diamond Packaging in 2007.
Shorewood Packaging in 2009 added TerraGreen to its greenchoice Environmental Solutions program. The water-based coating with a formulation based on natural resins and waxes allowed easy recycling of coated paper board packaging.
Keller Crescent developed a process using special inks to reduce printing defects on recycled paper board. Customers could purchase recycled product with quality comparable to high-quality SBS board at $5 to 15% cost reduction. Keller Crescent met customer preferences for low volatile organic compound emissions, and found slower speed production was no always an issue. “Many of our smaller private labels runs are more accepting of sub-speed issues. It becomes a lot easier for many of these OTC drugs to move to recycled packaging and maintain a green initiative,” said Bob Papa, then vice president, operations, Keller Crescent Co. in Portland, CT in 2010.
Conclusion
OTC sales have grown from $13.3 billion in 1993 to $29.3 billion in 2012, with Rx-to-OTC switches helping to fuel the growth, according to the Consumer Healthcare Products Association.
The OTC category would appear to have bright future given the momentum toward self-care, efforts to reduce health care costs, and FDA’s recent push to make OTCs more available.
With the NSURE concept (Nonprescription Drug Safe Use Regulatory Expansion) introduced in 2012, the agency is looking to address the under-treatment of common diseases by allowing prescription drug products to be available as non-prescription.
FDA has envisioned that various technologies could support the model such as kiosks in pharmacies or questionnaires on the Internet to help consumers properly diagnose a health condition and select a drug product. Special conditions would apply to types of nonprescription products such as requiring a talk with a pharmacist or having a diagnostic test.
“OTC drugs have had great success in providing consumers with excellent self-care options. But our concept of self-care is limited to conditions that can be self-diagnosed and self-treated based on the information in the drug facts box, combined with common knowledge,” said Janet Woodcock, M.D., director, Center for Drug Evaluation and Research, FDA.
“What we are asking is, should there be more flexibility in the concept of nonprescription drugs? Can we broaden the assistance a consumer gets and increase the types of medicines that might be available over-the-counter,” she added.
Next page: Milestones in over-the-counter packaging
Milestones in OTCs
1999--FDA issues the OTC “Drug Facts” label regulation, with new format and content requirements covering most OTC drug products.
2004--An FDA rule establishes across-the-board content and warning labeling for thousands of orally ingested OTC drugs that contain certain levels of calcium, magnesium, and potassium.
2007--In accordance with the Dietary Supplement and Nonprescription Drug Consumer Protection Act of 2006, FDA expands post-market adverse event reporting requirements on OTCs to include besides OTCs approved through NDAs and ANDAs also product marketed under an OTC drug monograph process. Additionally, dietary supplements are required to implement reporting on adverse product events, and in manufacturing meet cGMP requirements.
2008--The Consumer Healthcare Products Association announces that OTC manufacturers will voluntarily adopt labeling changes for children’s OTC cough and cold products, with new labeling that says “do not use” in children under 4 years of age.
2009--FDA requires stronger and more comprehensive labels on pain relievers, warning of the potential for liver damage when using acetaminophen, and the potential for stomach bleeding with non-steroidal anti-inflamatory drugs (NSAIDS).
2009.--Pfizer acquires Wyeth gaining Wyeth Consumer Healthcare products in areas including analgesics, cough/cold/allergy, vitamin/nutritional supplements, and potential Rx-to-OTC switch candidates.
2009--Merck & Co. buys Schering-Plough Corp. in a $41.1 billion merger providing Merck with a sizable consumer products line with products including Claritin, Coppertone, Dr. Scholl’s,
2009--Sanofi-Aventis gains entry into the US OTC market and a portfolio of OTC drug and personal care products with the acquisition of Chattanooga, Tenn.-based Chattem.
2010--FDA hosts a workshop on “Developing Guidance on Naming, Labeling, and Packaging Practices to Reduce Medication Errors” toward developing guidance on the quality and dissemination of drug information and design of drug labels and product packaging.
2012—FDA seeks input on NSURE (Nonprescription Drug Safe Use Regulatory Expansion), a new paradigm for addressing the undertreatment of common diseases by making prescription products available as nonprescription. If adopted, drugs would be approved as OTCs, with the use of new technologies assisting patients and with conditions of safe use such as care giver assistance.
About the Author(s)
You May Also Like


