December 8, 2015
Salaries are healthy and job satisfaction is high for pharmaceutical and medical device packaging professionals, but respondents to our annual survey report increased workload and hiring constraints.
In 2015 the U.S. economy has shown signs of recovery, and respondents to PMP News’s 2015 Industry Outlook and Salary Survey are reporting some gains. “Business is growing with new products and market share,” writes one professional. “Business seems to be coming back and is on the rise,” shares another. And “we continue to grow at a good rate,” says yet another.
Nonetheless, both packaging department managers and employees speak of lingering constraints. These may be explained by ongoing mergers and acquisitions as well as the now-entrenched tendencies for businesses to operate as leanly as possible.
For instance, there’s “more pressure to do more while spending less,” reports one professional, who explains that his firm is “adapting to changing customer needs for smaller, faster, cheaper options.”
“The economy has resulted in additional work without additional head count,” reports another.
Some respondents report that salaries in the industry are being impacted. “As the economy recovers, the prices go up on everything and the salary stays the same,” reports one.
And there may be an impact to packaging departments and therefore organizations. One manager sees “no impact as an employee,” but “from a hiring perspective, though, it’s difficult to hire packaging engineering staff right now. I attribute this to people being concerned with stability and don’t want to move around.”
Reports another manager: “As the economy improves,
the opportunities for employees continue to expand. Maintaining employees under
these conditions becomes a greater challenge. Employee turnover is disruptive, expensive, and places an increased demand on the organization.”
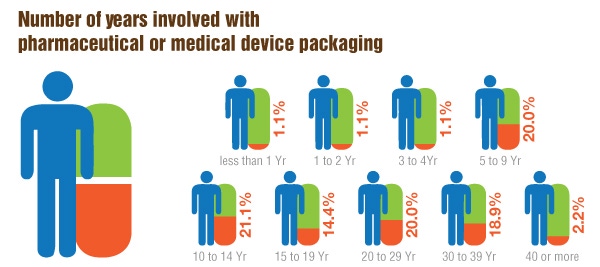
The numbers tell a slightly different story. Annual salaries and raises in the pharmaceutical and medical device industries, interestingly, are averaging much higher in this year’s survey compared with those in last year’s. According to this year’s group of respondents, the average annual salary is $126,300, compared with last year’s $115,000. Raises this year are averaging 4.3%, compared with 3.5% from last year. Because our survey is anonymous, it is impossible to know whether the higher salaries this year are true gains from last year, or just an entirely different group of respondents with different circumstances. What is similar, however, is that year after year, most packaging professionals in these industries appear to be content—80% this year are either satisfied or very satisfied with their current positions, compared with 69.3% last year.
M&A AREN’T GOING AWAY
Several respondents report that their personal compensation has been affected by mergers and acquisitions.
“Many pharmaceutical companies are merging, which can equate to loss of many jobs,” says one packaging professional. “My company has just gone through this.
A “merger buy out” has resulted in one respondent’s position being eliminated; others report positions becoming “redundant.”
The results, however, are varied, and may in fact increase options for qualified packaging engineers. “Hard to retain employees at times due to M&A, layoff issues, good job opportunities for packaging engineers,” explains one manager.
Says one employee: There’s “more competition between companies for the same type of workers, i.e., medical device and packaging engineers.”
Another employee believes that “it will take ever increasing compensation (maybe perks) to attract and keep highly skilled and experienced personnel.”
LEAN AND MEAN?
But economic growth does not necessarily bring the resources to scale up to meet new demand, it seems. One manager reports that there is “more work with [fewer] resources to perform it.”
Reports another manager: “My team has been affected by the economy over the last year. My team has to take on additional responsibilities to remain efficient due to limited personnel. I am unable to hire new employees at this time because of budget restraints.”
Says another: “We have to ask for more with the same salary. No additional resources.”
According to the numbers, nearly
one-third of responding packaging professionals this year are working 50 hours or more each week.
“Middle [management] and down experience huge budget cuts and ‘leaning out’ while CEO pay and cash acquisitions skyrocket,” reports one respondent.
There’s “pressure for cost-cutting measures and flat salaries for our employees,” says one manager.
Says one packaging professional: “I work for a startup company and the available capital for funding small companies was difficult to obtain. As a result, as employees we received no raises over the last 3 years and had 20% of salary kept in escrow for over 1.5 years.”
While one might take these as extreme examples, given the higher average annual salary of $126,300 (compared with last year’s $115,000), the average annual salaries by industry tell a slightly different story. Last year, the figures were fairly close: $115,100 for packaging professionals working for a pharmaceutical manufacturer, and $116,900 for those working for a medical device manufacturer. This year, however, packaging professionals working for a pharmaceutical manufacturer are the ones coming out ahead, averaging annual salaries of $138,200, compared with $110,300 for medical device packaging professionals.
Professionals in R&D and package design average salaries of $140,900, compared with $116,200 in engineering and $115,800 in production.
Nonetheless, there may not be guaranteed employment opportunities. Some managers are turning to “temp workers over full time,” says one.
If temporary help becomes the norm, it’s hard on both employee and manager. “It’s difficult to train somebody that is not permanent and then you have to let them go and start all over again,” says one manager.
Morale can suffer. “Similar to before—it is difficult to maintain great morale when you can not increase production personnel salaries,” says another.
Such conditions foster an approach that one manager describes as “careful about the pennies.”
Outsourcing and offshoring also appear to impact long-term prospects for some respondents. One says “outsourcing will make it difficult to find work with benefits and that lasts more than 6 months.”
Another reports that “offshoring will eliminate my job by year end if not sooner and result in my need to find a new job, which may not provide similar compensation.”
And another shares that he or she is being affected by “moving manufacturing to Mexico.”
And one manager reports: “Transition of offshoring has resulted in reduction/elimination of temporary employees.”
One employee reports “lots of contract and foreign employees added,” and says that “management knows there are no jobs, so they ask crazy hours.”
REGULATIONS
Regulatory compliance does appear to prompt investment and hiring. When asked what is likely to influence compensation, one employee points to “regulations and inspection. Any regulatory requirement/hurdle does resonate on the leadership’s ears and they staff and compensate accordingly.”
When asked what regulations and/or industry standards
are presenting new challenges, several respondents point toward serialization requirements around the world, FDA’s Unique Device Identification, updates to U.S. Pharmacopeia chapters, EU Good Distribution Practices, and interpreting shipping test requirements.
Several respondents also criticize the medical device excise tax provisions of the Affordable Care Act. “Medical Device excise tax is taking needed money for development of new products and product improvements,” says one professional.
Another professional says that he or she has “seen the loss of countless lifesciences jobs due to the medical device tax.”
“Medical device tax continues to require us to do more with less and has prevented us from acquiring additional staff,” adds another.
Another is hopeful: “Medical device tax repeal is likely to have a significant impact by putting raises back to their former percentages.”
One professional points to other market challenges, namely “medical insurance reimbursements. FDA clearance was hard enough, but reimbursements is more difficult and extends [our] timeline to get a start-up in making money and therefore survive!”
QUALITY
Packaging professionals report some efforts toward quality improvements, which could also prompt investment in departments.
“We have only refocused our priorities on quality improvements, but not directly impacted by the economy,” says one.
Another reports “quality improvements to avoid/eliminate field actions.”
OPPORTUNITIES
Despite increasing regulations, increased offshoring, and the new lean approach, packaging professionals in the pharmaceutical and medical device industries are pleased with their industries, their companies, and their positions.
Perhaps it’s because of respondent statements like this one: the “pharmaceutical industry has relatively higher wages than average job in the market.”
But opportunity is for the qualified. This same respondent says: “we can afford to be a little more selective with skill sets.”
And survey respondents express the need for qualified personnel. One says the biggest challenge in developing a new package is “finding employees with [the] skill set to do it.”
Losing qualified employees seems to be an issue, too. For instance, when working with existing packaging equipment, respondents report challenges “ensuring repeatability as a result of employee turnover.”
Challenges such as serialization are bound to create ongoing opportunities for skilled and experienced professionals. For instance, one respondent reports a “lack of in-house technical expertise,” so companies such as this one will certainly need to address it in some manner.
And there’s always work to be done: “Biggest challenges are always from interpretations of existing standards, primarily ISO 11607, and primarily issues from European regulatory bodies,” says one professional.
Another points toward “FDA, the constant expansion and evolution of requirements. We are never sure what they want, but you can be sure it is always more.”
METHODOLOGY
Data and verbatim responses were obtained during an online survey of professionals in pharmaceutical and medical packaging. Between June 22, 2015, and July 13, 2015, e-mails were delivered to a total of 13,035 individuals inviting them to complete a Web-based survey. The survey was closed with 178 total responses, a 1.4% response rate.
The results presented in this article are based on the 90 respondents who indicated that they work full time at organizations best described as one of the following: a manufacturer of medical devices, pharmaceuticals, biologicals, in vitro diagnostics, or nutritional supplements. Respondents who appeared to work outside these organizations were manually removed from the results before final tabulation.
Respondents were allowed to skip certain questions; in this article, we have provided the number of respondents answering certain questions.
About the Author(s)
You May Also Like




