What You Need to Know About USP 661 Series on Plastic Packaging
With an implementation deadline less than two years away, pharmaceutical manufacturers need to get up to speed quickly on the nuances of the USP <661> general chapter series for Primary Plastic Packaging testing.
At a Glance
- Plastic articles used to package medical products must be assessed against requirements in the <661> chapters
- The industry faces challenges when testing blister packaging systems
- Some interpretation is involved when comparing FDA’s guidance for packaging human drug and biologics with <661> chapters
The USP-NF is a combination of two compendia: the United States Pharmacopeia (USP) and the National Formulary (NF). When USP-NF set out to revise General Chapter <661>, Containers-Plastics in 2013 [published in Pharmacopeial Forum/PF 39(5)], one stated goal was to increase flexibility by going from prescribed tests and limits to a model where the drug manufacturer could apply tests based on risks associated with the packaging’s Materials of Construction (MoC) and the drug product dosage form. This involved going from one chapter <661> to a suite of chapters (log-in required for the links):
• <661> Plastic Packaging Systems and Their Materials of Construction
• <661.1> Plastic Materials of Construction
• <661.2> Plastic Packaging Systems for Pharmaceutical Use
• <1661> Evaluation of Plastic Packaging Systems for Pharmaceutical Use and Their Materials of Construction
These chapters have gone through many USP revision cycles with opportunities for stakeholder feedback. The published <661.1> and <661.2> chapters now have an official implementation date of December 1, 2025, with early adoption permitted.
Savushkin / iStock via Getty Images Plus
Do these chapters apply to packaging for my products?
As outlined in the USP General Notices 3.10, compendial content are “made applicable to an article through reference in General Notices, a monograph, or another applicable general chapter numbered below 1000.” While <661> chapters are not directly referenced in drug product monographs, the USP General Notices 10.10 states that “All articles in USP or NF are subject to the packaging and storage requirements specified in Packaging and Storage Requirements <659>.” Within <659> are these stated requirements:
“Any plastic material used to construct a Packaging system must meet the applicable requirements of Plastic Materials of Construction <661.1>. All Packaging systems must meet the applicable requirements specified in … Plastic Packaging Systems and Their Materials of Construction <661>, Plastic Packaging Systems for Pharmaceutical Use <661.2> …”
Therefore, the <661> chapters must be assessed for their application to plastic articles (materials, components, and systems) used to package medical products (pharmaceuticals, biologics, and dietary supplements). The “articles” to which the chapters apply are primary packaging, which have contact with the dosage form provided to the patient, and potentially interacting materials (see <1661> for the concept of non-interaction materials). The holder of the drug product application and drug product manufacturer bear primary responsibility and accountability for ensuring that the requirements of the chapters are met.
Early versions of <661.1> and <661.2> included a “grandfathering” clause that exempted plastic packaging that had been previously approved by a regulatory authority. This exemption was removed.
Early versions of <661.1> and <661.2> included a “grandfathering” clause that exempted plastic packaging that had been previously approved by a regulatory authority. This exemption was removed (at the request of the Food and Drug Administration). Therefore, the chapters also apply to marketed product where USP requirements must be met.
Applying <661.1> to plastic packaging Materials of Construction (MoC).
Chapter <661.1> provides tests for nine (9) different MoC used for drug product’s packaging, and guidance for unaddressed materials. Such testing is useful when selecting the appropriate packaging for a medical article/drug product because it provides greater confidence to the drug manufacturer when selecting and qualifying packaging that must meet the requirements of 661.2.
Requirements outlined in <661.1> Table 1 associate a higher degree of testing to packaging for dosage forms other than Oral and Topical. Testing begins with the preparation of solutions (such as S1, S2, as per USP’s naming convention) extracted from the MoC used to make the packaging component. Material or solutions are then submitted for identification, physicochemical tests, and additives quantitation against USP standards.
Chapter <661.1> provides a mechanism that can be adopted by the packaging supplier to demonstrate that the components they produce align with USP requirements, therefore providing a marketing edge over competitors that have not evaluated their component materials against <661.1>.
Applying <661.2> to plastic packaging systems.
While testing or certification per <661.1> supports that the MoC used for packaging is suitable for drug product packaging, the scope of chapter <661.2> addresses packaging components and systems.
Per the Scope of <661.2>:
“The requirements of <661.1> are met by performing the tests in <661.1> or if the material is used in a packaging component or system that meets the requirements of <661.2>. A product’s packaging component or system is deemed chemically suited for its intended use, if it meets the requirements in <661.2>.”
Table 1 in <661.2> exhibits the intended flexibility of testing packaging systems according to the risk of interaction between drug product form and the container closure system (CCS).
Canva
Physicochemical tests are expected for all applicable plastic packaging components and systems. Drug product dosage forms other than oral or topical are required to meet USP-NF bioreactivity chapter <87> Biological Reactivity Tests, In Vitro; whereas tests for chemical suitability for use (such as extractable or leachable testing) are at the discretion of the drug product manufacturer based upon risk. Tests for identification and quantitation of unknown chemicals are required when a sample tested exceeds limits (that is, Total Organic Carbon (TOC) or Absorbance).
Flexibility is incorporated within chapter <661.2> when preparing test solution (C1):
• The solvent (water) can be filled into the packaging.
• Four temperature/time combinations are provided for the extraction process.
• The contents of many individual systems may be combined to achieve the desired volume for analytical testing.
The test solution (C1, for example, as per USP’s naming convention) volume is based upon the applicable tests. Per the authors’ experience, at least 150 milliliter (mL) is needed and should be doubled if there is a potential need for chemical suitability assessment(s).
It is not directly stated in the USP, however, for practical purposes, it is not necessary to perform <661.2> tests on all shapes and size variations of packaging systems made with the same MoC. It may be justified to test a combination that represents the highest risk — such as choosing those with a higher surface area-to-volume ratio while maintaining the appropriate scale.
Challenge of testing blister packaging systems per <661.2>.
The testing of blister packaging systems is a challenge the industry faces when implementing <661.2>. Even with the variations noted above, the following issues exist:
• Delamination between the lidding and base layer can occur (see Figure 1).
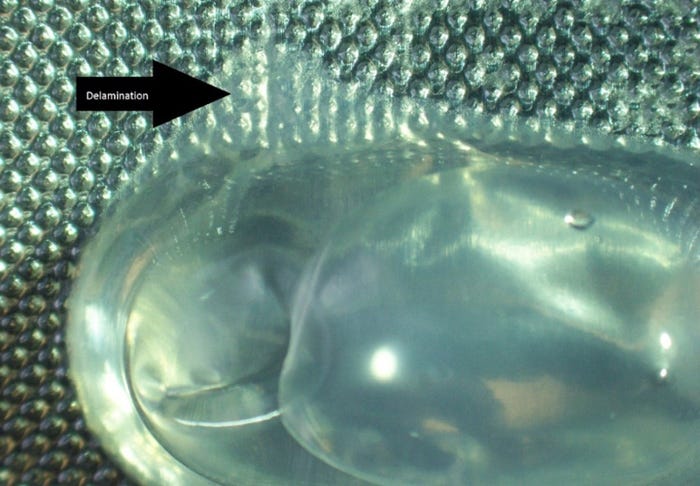
Figure 1 - Photo shows a delaminated blister during preparation of solution C1. Eli Lilly and Co.
• Volumes needed for testing (such as 150 mL) can be difficult to obtain using the common blister cavity (such as 0.8 mL). Though a larger blister mold could be created and less cavities filled, the effort may be cost prohibitive.
• Foil lidding for blisters is often made with multilayer components, and only one side has product-contact. Therefore, the option of extracting such components in an inert container would not provide representative results.
• Sealing blisters with non-product material (water) may require an alternate filling line.
• The process of removing the prepared C1 solution after incubation creates risks for contamination.
Packaging suppliers and <661>.
As noted above, it is in the best interests of packaging suppliers and drug product manufacturers to test component MoC according to <661.1>. However, certain limitations exist for those companies that manufacture such components.
The manufacturing of plastic components involves heating resin and molding or extruding it into desired shapes. Such activities inherently produce some levels of charred carbonaceous material on the surface of the produced film or container. When components are tested, the carbonaceous material may cause a TOC result that exceeds applicable limits in <661.2> (that is, 8 to 32 milligrams/Liter based upon component volume).
Ideally, packaging manufacturers will receive confirmation from their resin suppliers that it has been tested against <661.1> and certified to meet requirements. However, even with this confirmation, the produced plastic component could exceed limits for TOC or ultraviolet (UV) absorbance. This leads to the identification and quantification of the chemicals responsible for the out-of-limit result to show that the probable risk posed by all the chemicals, considered individually, is within acceptable parameters.
Packaging manufacturers that perform component testing using <661.2> (such as in an inert vessel) have the advantage of already knowing key information, such as additives or processing aids. Such information would be beneficial to drug manufacturers seeking to meet <661> requirements when addressing any test results that exceed acceptance criteria.
It is imperative drug product manufacturers and packaging component suppliers work together to ensure drug product packaging is qualified for its intended use.
It is imperative drug product manufacturers and packaging component suppliers work together to ensure drug product packaging is qualified for its intended use. This requires sufficient knowledge of the composition of materials used, such as additives, to drive any testing (such as extraction studies) necessary to support any chemical suitability for use requirement in <661.2>.

Burton0215 / The Image Bank via Getty Images
Testing laboratory considerations.
Many drug manufacturers and packaging component suppliers will rely on external laboratories to confirm compliance with the <661> chapters. This is because such laboratories can focus resources and expertise on the complexities related to <661.1> and <661.2> testing requirements.
Considerations when using an external laboratory should include:
• Ensure labs have expertise in testing and interpreting results per <661> chapters.
• Clearly describe the MoC to the lab, including known additives.
• Communicate sample preparation details, whether the lab will prepare samples, or they have been prepared before being submitted. For example: specify how to extract water from containers before sampling (such as clean exterior, clean cutting, dust-free environment, gloves, pour vs withdrawal from container).
• Understand the lab’s process for handling out-of-specification (OOS) results (such as when retesting is acceptable).
How are the <661> chapters reflected in regulatory requirements?
It is common for drug product manufacturers to cite <661> when they register products packaged with plastic containers. Chapter <661> addresses such commitments by stating that “A product’s packaging component or system is deemed suited for its intended use, if it meets the requirements in <661.2>.”
Chapter <661> also references the application of 21 CFR Part 174 — Indirect Food Additives: General regarding plastic packaging and their additives. This aligns with guidance from the Food and Drug Administration (FDA) that clarifies requirements for plastic packaging (also referred to as container closure systems): FDA Guidance for Industry Container Closure Systems for Packaging Human Drug and Biologics-1999.
When comparing this FDA guideline with <661> chapters, some interpretation is involved.
When comparing this FDA guideline with <661> chapters, some interpretation is involved. The FDA guidance outlines suitability based upon the route of administration and the dosage form; see Table 1 (page 6; or page 9 of the PDF), Examples of Packaging Concerns for Common Classes of Drug Products, and Table 2 (page 12; or page 15 of the PDF), Typical Suitability Considerations for Common Classes of Drug Products. The FDA testing guidance for those drug product forms with Highest/High Degree of Concern and High/Medium Likelihood of Interaction align well with testing outlined in <661.1> and <661.2>. However, this is not the case with Low Degree of Concern and Low Likelihood of Interaction (that is, Solid Oral Dosage Forms - SODF).
For example, per the guidance, the risk of interaction between plastic packaging components and SODF (such as tablets, gelatin capsules) is small. Requirements depend upon whether the SODF is sensitive to moisture. For such dosage forms, this guidance includes the tests for Polyethylene Containers in the USP <661>. However, it does not reflect testing per the recently published <661.1> and <661.2>. The FDA guidance was issued in 1999 and has not been updated in parallel with USP.
Additionally, per the guidance, the appropriate indirect food additive regulation is typically considered sufficient evidence of safety for SODF packaging.
It is recommended that drug manufacturers consult with regulatory authorities to appropriately interpret the requirements applicable for their products.
Therefore, it is recommended that drug manufacturers consult with regulatory authorities to appropriately interpret the requirements applicable for their products.
Summary.
The <661> chapters must be assessed for their application to each medical article’s primary packaging. Chapter <661.1> is applicable to determine the suitability of the packaging component’s Material of Construction (MoC) (such as during packaging development for the product). Chapter <661.2> is required to be applied to primary packaging systems to comply with <661>. It incorporates flexible sample preparation options; however, challenges still exist with the testing of blister packaging systems.
As pharmaceutical companies work to align their packaging processes with USP <661> suite of chapters and existing regulatory commitments for medical articles, we need to be mindful of the complexities and time required to establish testing plans, arrange testing laboratories, and document suitability of packaging systems. Therefore, it is imperative that industry address these challenges before the chapters are official on December 1, 2025.
Abbreviations used in this article:
C1 – Test Solution for 661.2, specific to packaging systems/components, as per USP naming convention
CCS – Container Closure System
FDA – Food and Drug Administration
mL – Milliliter
MoC – Materials of Construction
OOS – Out-of-specification
PF – Pharmacopeial Forum
S1, S2 – Test Solutions for 661.1, specific to materials, as per USP naming convention
SODF – Solid Oral Dosage Forms
TOC – Total Organic Carbon
USP-NF - United States Pharmacopeia and National Formulary
UV – Ultraviolet
About the Author(s)
You May Also Like








