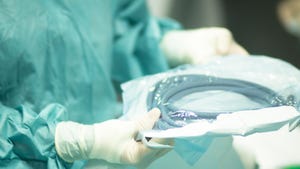
Medical Packaging
Dordan Manufacturing cleanroom Aric Slavin
Medical Packaging
Medical Thermoformer Achieves ISO 13485 CertificationMedical Thermoformer Achieves ISO 13485 Certification
The family-owned business reinforces its commitment to quality and evolving regulatory requirements.
Sign up for the Packaging Digest News & Insights newsletter.






















.png?width=300&auto=webp&quality=80&disable=upscale)




.jpg_(1).png?width=300&auto=webp&quality=80&disable=upscale)





.png?width=300&auto=webp&quality=80&disable=upscale)




