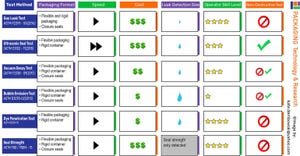
Testing
Sanguina AnemoCheck Home at-home anemia test
Medical Packaging
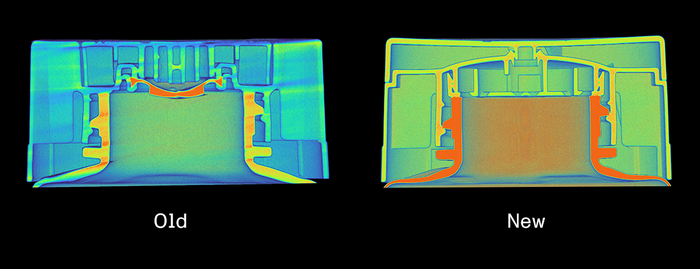
Medical Test Kits Find a New HomeMedical Test Kits Find a New Home
Patients appreciate the comfort, convenience, and confidentiality of testing and treating themselves where they live.
Sign up for the Packaging Digest News & Insights newsletter.