Essentra to offer labeling solutions at Pharmapack North America
November 19, 2015
The pharmaceutical market is facing a number of ongoing challenges, and Essentra believes its packaging business unit can offer a number of solutions. The global packaging company, which completed its acquisition of Clondalkin Specialist Packaging Division at the end of January, will be showcasing its capabilities in Booth 4003 at the upcoming Pharmapack North America June 9-10 in New York City.
“As patient demographics shift towards an older population, prescription regimes are becoming more complex—this means that the industry must explore new ways to help maximize the efficiency of drug information delivery to ensure patient safety,” explains Malcolm Waugh, Group Commercial Director, Essentra plc.
At the same time, the pharmaceutical sector and related packaging industries are facing many changes in the face of strict requirements for quality and change control, Waugh says. “The structural changes that have occurred within the industry all focus on lowering costs— from a shift to generics to the cost savings expected from the consolidation of end-market players,” Waugh says. “At the same time, the governments continue to increase regulations associated with packaging, from tamper-evidence to track and trace to the addition of electronic patient information leaflets for use in private structures and hospitals where caregivers and will have access to real-time updates on drug information.”
Essentra is working to provide support through “increasing the formats and features available on our cartons and labels to providing innovative ways to maximize the amount of information available to patients,” he says. It is also developing a “diverse range of solutions from just the basics to enhanced features to support the cost profile requirements of generics to the more consumer-centric OTC packaging.”
For instance, Essentra’s Plurium leaflet that features a foldable booklet and offers the ability to present data, drawings, graphs, and text via increasing the printable surface area, Waugh says. �“The easy-to-read booklet style format is still easy to fold and small in size.”
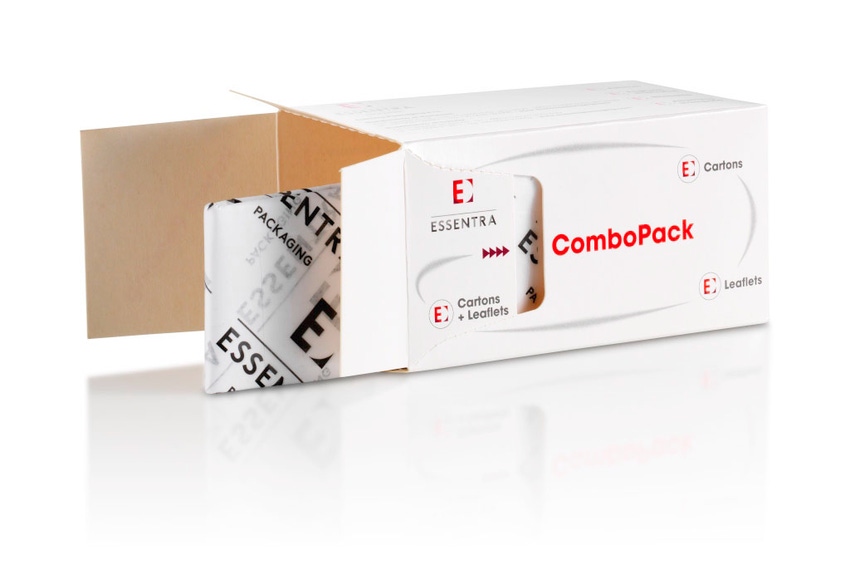
In addition, “Combopack delivers a pre-combined carton with literature for applications where high-speed literature-leaflet insertion is not appropriate or desired.” Essentra is reporting that “carton volumes are increasing in the USA as the market is beginning to transition to the use of blister packs to encourage proper patient dosing through packaging rather than by instruction on a pill bottle,” Waugh says.
Pharma companies are also looking for advanced features. “Consumer-oriented packaging features for the OTC market are an increasing request from customers as drugs transition to the front of the counter,” he says. “Features such as glossy varnishes, hot-foiling, and embossing—anything that will help differentiate the product on the shelf are becoming more relevant as certain prescriptions move to OTC where the consumer chooses the right product for themselves.”
“We also offer options that allow for digital information to be accessed by consumers via QR codes applied to secondary packaging,” Waugh adds.
At Pharmapack North America, Essentra will be highlighting both Plurium and Combopack along with its serialization capabilities.
“Driven by regulatory requirements in certain markets—particularly export markets—customers are increasingly requesting serialization for use in product tracking,” Waugh says.
Essentra’s “global footprint” allows the company “to offer a range of solutions to customers on an international basis with consistent quality and service,” he says. “We are also offering Vendor Managed Inventory to deliver supply chain efficiencies as well.”
About the Author(s)
You May Also Like




