Package engineering considerations for medical device sterilization

Package design is an integral part of the entire medical device development process, yet it is frequently left as an afterthought or the decision is made to purchase an off-the-shelf system without consideration to the device or sterilization method.
The first step in the packaging design journey generally starts with choosing the appropriate packaging materials. This can depend on the geometry of the device, how it will be used in the field and the selected sterilization method. The package must demonstrate that it is effective in facilitating sterilization, maintaining sterile barrier properties for the claimed shelf life, physically protecting and containing the product, and facilitating easy removal and use in the field. Design and subsequent testing to meet and prove these criteria can be a time consuming and involved process.
While all of these functions are critical to the success of the packaging, facilitating sterilization and maintaining sterile barrier properties are probably the least understood. Let’s take a look at some of the most commonly used forms of sterilization, design features and a few test options that can help determine an optimal packaging system.
Though there is a large list of sterilization methods to choose from, there are three common methods which tend to dominate the industry: ethylene oxide (EO), ionizing radiation (otherwise known as gamma radiation or electron beam) and steam sterilization. Each device, sterilization and design combination can present different challenges. For example, EO may have trouble reaching the center of the lumens for devices with long lengths of tubing, or devices with sensitive electronics may not fare well with gamma radiation or electron beam sterilization. All of these aspects need careful consideration in selecting the correct materials.
Packaging engineering considerations for EO sterilization
EO is the most common sterilization method used in the medical industry and accounts for more than half of all sterilized medical devices. EO is a colorless, flammable and carcinogenic gas that is primarily used as a precursor to polymers and products like antifreeze. The process for sterilization includes preconditioning the products, exposure and then aeration (off gassing).
Preconditioning involves exposing the product to a warm, humid environment until a uniform internal temperature and humidity is reached (about 45-degrees C and 55% to 65% relative humidity). Products are then loaded into a sealed chamber where they are exposed to EO gas. After a predetermined and validated exposure time, they are removed and allowed to off-gas at elevated temperatures to remove residual EO. This form of sterilization is known as a gas-in/gas-out process.
The EO gas needs the ability to enter the packaging to come into contact with the device, and gas needs to be able to exit the packaging afterwards to reduce toxicity levels. Therefore, one of the primary design considerations when using EO is the permeability or breathability of the packaging materials.
The selection of porous material options are widely available and the material chosen can contribute to the overall processing time and cost. Common materials used are spun bound high-density polyethyelene (HDPE)—Tyvek is a popular example—and medical grade papers. The more breathable the materials, the more easily EO enters and exits the package. Consideration of pressure changes throughout the exposure cycle is also important. The package seals will need to be assessed for appropriate strength to demonstrate the ability to withstand the pressure changes throughout the cycle—but the strength of the seal should not be so strong that it impacts the capability of the end user to open the package and access the device.
One of the best tests for evaluating the breathability of packaging is ISO 5636-5 Determination of Air Permeance. This test uses a device known as a densometer, which uses a cylinder pulled downward by gravity to pass a known volume of air through the porous material. The more porous the material, the more quickly the cylinder falls.
Example of spun-bound HDPE packaging that fares well in EO sterilization.
The use of burst testing, ASTM F1140 Standard Test Method for Internal Pressurization Failure Resistance of Unrestrained Packages, can provide valuable data for the evaluation of seal strength when packaging is exposed to pressure changes. This test works by first sealing any porous areas of the package (either using common packaging tape or other methods), and then air is introduced to the interior of the package at a controlled rate until the weakest seal fails or pops. Information regarding the failure location can help engineers determine possible design issues which may occur after exposure to EO cycles.
Packaging engineering considerations for radiation sterilization
The next most prevalent form of sterilization is ionizing radiation—gamma radiation and electron beam. The sterilization process used for ionizing radiation sterilization is different and simpler from gas-based methods. During gamma radiation, packaged product is loaded into totes that ride a conveyor system. The conveyor then transports the product around a radiation source of cobalt 60 until the appropriate dose of radiation has been applied.
One of the key characteristics of ionizing radiation is that it has a tendency to fundamentally change materials on a molecular level. One of the most common forms of change is known as scission. Scission occurs when a long molecule chain is caused to be broken into smaller segments. If you imagine most polymers as a large knot of long molecules, and then the molecules are cut short: the physical characteristics of the knot are going to change. Often this causes optical changes (change of color, opacity and gloss), as well as physical changes (creates brittleness).
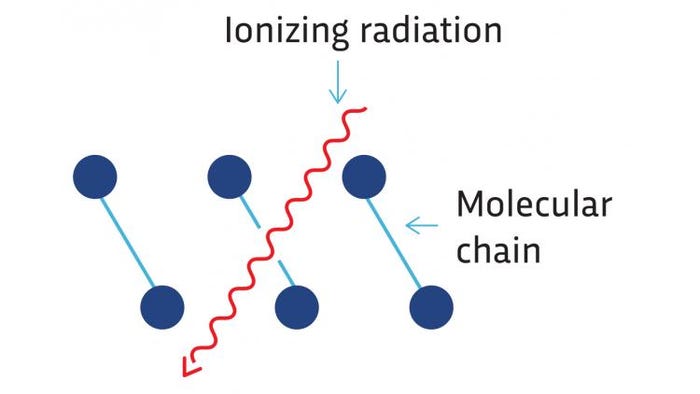
Molecular scission resulting from ionizing radiation.
Both porous and non-porous materials are equally compatible with radiation sterilization, which provides more variety in packaging materials than gas-based methods. However, because ionizing radiation can cause materials to become more brittle, the package design should select materials that demonstrate appropriate strength characteristics after sterilization.
Testing for these types of material changes can be accomplished using several test methods.
Seal strength testing is the most predominant method used to evaluate these types of material changes and can be accomplished following ASTM F88 Standard Test Method for Seal Strength of Flexible Barrier Materials. The seal strength test evaluates the ability of the package to both contain the product and identify the amount of strength it takes to open the package. Imagine trying to open a gamma sterilized package containing a medical device in an operating room environment. Rather than having the package open at the seal as designed, you have material failure and the packaging opens by rupturing within the film itself. This could cause the medical device to fall onto non-sterile surfaces and become unusable.
Packaging engineering considerations for steam sterilization
Steam sterilization (wet heat) is the most common form of non-industrial-based sterilization. During this process the packaging is placed within a chamber that is filled, under pressure, with steam (see photo at top of article). Sterilization usually occurs at either 121 or 132 degrees Celsius. This process is similar to gas-based processes such that packaging for steam sterilization must be porous and allow free passage of the steam. Common packaging materials for this method of sterilization are usually paper-based pouches or woven and non-woven wrap materials; reusable metal trays are also a favorable option.
A significant item for consideration when using steam is to evaluate the interaction of the moist heat with adhesives that are used to bond trays to lid materials. If the adhesive is not designed to withstand high temperature and moisture, the sterile barrier could be compromised.
There are multiple test options available for the evaluation of steam-sterilized packaging and the correct selection should be based on the characteristic needing to be evaluated. Premature bursting of the packaging or streams of bubbles emanating from the seals of the packaging may indicate a compromised adhesive layer. Seal peel testing may also be appropriate before and after sterilization to determine if acceptance criteria is still met, and to ensure that detrimental changes to the seal strength have not occurred.
Conclusion
The information presented in this article should provide a good starting place for medical device manufacturers, package designers and sterilization engineers when choosing packaging solutions that are appropriate for the given sterilization method. It should illuminate possible modes of failure and ensure that detection is made to assist with redesign if needed.
Any sterilization process will affect materials and packaging to some degree; the goal when evaluating packaging considerations by sterilization method is to understand how detrimental the changes are and how to successfully mitigate them. More often than not, packaging design for the medical device industry is a cross-disciplinary exercise involving engineering, material science, sterilization (microbiology) and regulatory understanding. Gaining insight into these areas will greatly assist in timely packaging validation and approval.

Co-author Wendy Mach RM (NRCM), CQA (ASQ), serves as consulting manager for Nelson Laboratories LLC, a Sotera Health company, which is a leading provider of microbiological testing and consulting services for MedTech companies. Mach has more than 24 years of medical device manufacturing and laboratory experience. In her role as consulting manager, she works with clients to address their unique needs to meet the requirements for packaging validations. She is a participating member on the Technical Committee ISO/TC 198 Sterilization of Healthcare Products, the subcommittee chairperson for ASTM F02.15 Chemical / Safety Properties, and is a member of the Packaging and Distribution Expert Committee for United States Pharmacopeia (USP).

Co-author Andrew Manrique, B.S., CPLP (ISTA), packaging engineer for Nelson Labs, delves into the world of GMP (Good Manufacturing Process), GLP (Good Laboratory Practice) and many other areas compliant with Code of Federal Regulations Title 21. This, combined with his experience in Lean Manufacturing and statistical analysis, allows Manrique to better understand how the medical device qualification, verification and lifecycles are successfully implemented.
About the Author(s)
You May Also Like




