Diamond powder: A shiny new tool in drug packaging authentication
November 12, 2015
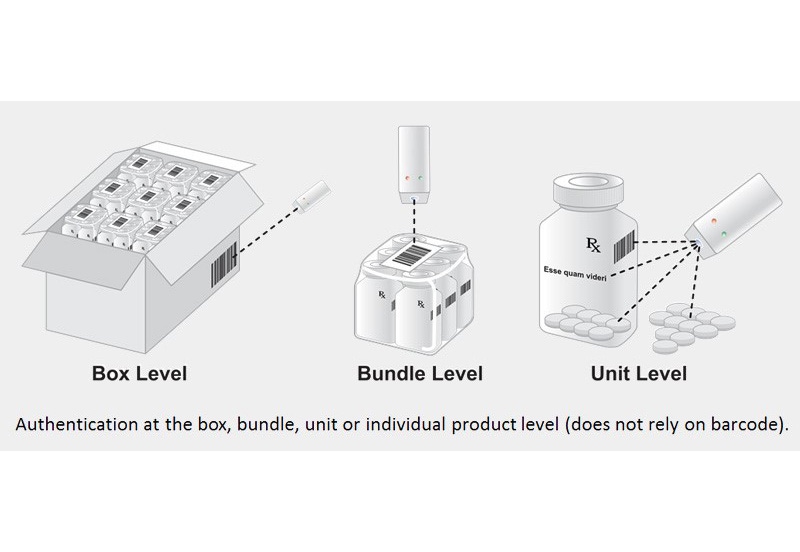
Counterfeiting has become everyone’s problem, stated Pfizer’s director of global security at Pharmapack North America earlier this year. Now a specialized research company is offering a shiny new solution: Diamond powder.
According to Taaneh, a company that specializes in drug authentication, global sales of counterfeit drugs are on the rise, citing a World Health Organization statement of $431 billion in a Council of Foreign Relations report. Current counterfeit protection technologies use serialization, color-coding, or imprinting methods, but Taaneh states these strategies can be easily replicated and can often only trace either product or package, not both.
CEO Andy Janoff, Ph.D., says that researchers are now exploring the use of diamond as an authentication tool for drug manufacturers.
“As an allotrope of carbon, diamond is an inert substance that can be added to drug formulations,” Janoff says. “It can also be added to packaging materials and the inks used in product labeling. When diamond is exposed to certain wavelengths of light, it emits unique spectral signatures that cannot be duplicated. Even in trace amounts, the spectral signatures occur and can be detected and confirmed with a programmable handheld scanner.”
Janoff says that using diamond in product and packaging makes it possible to authenticate a product at any point in the supply chain from manufacturer to consumer. Diamond powder is inexpensive and can blend easily into inks commonly used in labeling and printing, he says, adding that ink encrypted with diamond can be used to print already approved labels.
“There is no requirement for additional artwork or need for additional space,” Janoff says. “Adding diamond powder into industrial ink does not require specialized equipment and can be quickly and seamlessly integrated into most existing label and package production protocols. After the desired concentration of diamond powder is mixed into the ink before printing, all packaging and labeling processes can continue as usual without alterations or costly modifications to production procedures.”
Read the full article at Pharmaceutical & Medical Packaging News.
You May Also Like


