Novel drug-delivery devices merit coveted MDEA awards
Four user-friendly packages for drug-delivery and combination products help administer accurate doses and improve healthcare treatments. Eli Lilly and Co., Accord Healthcare Ltd., PharmaC LLC and Ethicon US LLC earned 2018 Medical Device Excellence Awards for their new products and developments.
Organized by media brand Medical Device and Diagnostic Industry MD+DI, the MDEA competition recognizes innovation in nine medical categories (see all winners here). One category, Drug-Delivery and Combination Products, relates directly to packaging and the winning developments are worth sharing with the Packaging Digest and Pharmaceutical & Medical Packaging News audiences.
In this category for 2018, MD+DI presented four awards: two gold awards, one silver and one bronze. Winning Gold are Eli Lilly and Co. and Ethicon US LLC. Accord Healthcare Ltd. took Silver. PharmaC LLC won the Bronze.
We start our descriptions of the winning packages with the Bronze Award winner and work our way up to the top Gold Award winners.
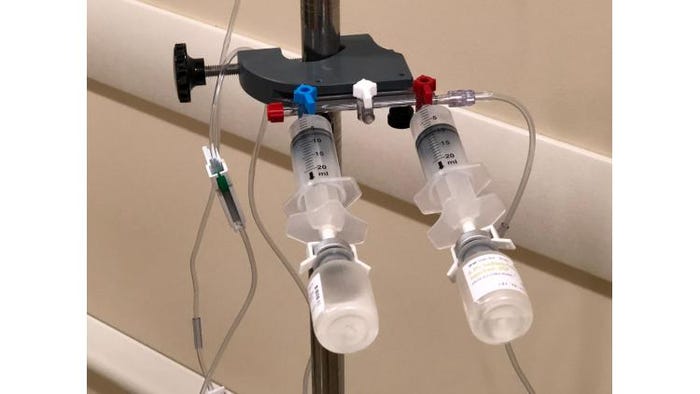
Bronze Award: OnSite-IV from PharmaC LLC
The OnSite-IV is a single-use device that facilitates creation of a sterile fluid path to transfer liquid from the syringe body to a vial for delivery into an IV bag or elastomeric device. The device provides both admixture and direct dispensing of medications in a single, closed system without the use of needles. Its universal design allows it to be used with any elastomeric device.
While intended to be used by healthcare professionals, OnSite-IV can be used in multiple healthcare settings: hospitals, doctors’ offices, nursing homes, military locations and even the patient’s home. The design functions similar to that of existing syringes—it requires a simple 180-degree rotation to operate—which minimizes training needed for users.
Preparing intravenous medications on-demand helps eliminate drug waste and addresses drug stability and transport issues, particularly for home intravenous use. OnSite-IV reduces shipping costs and concern with temperature control issues for homecare medications because drug stability is maintained until it is mixed and added to an IV container. The drug device also allows for greater use in locations where refrigeration is problematic, such as in third-world countries.
Because the medication vial can be attached to a manifold in an operating room setting, benefits for use in the OR include eliminating multiple aspirations and the need to label multiple syringes.
NEXT: Silver Award: Methofill (methotrexate) Self Inject from Accord Healthcare Ltd.
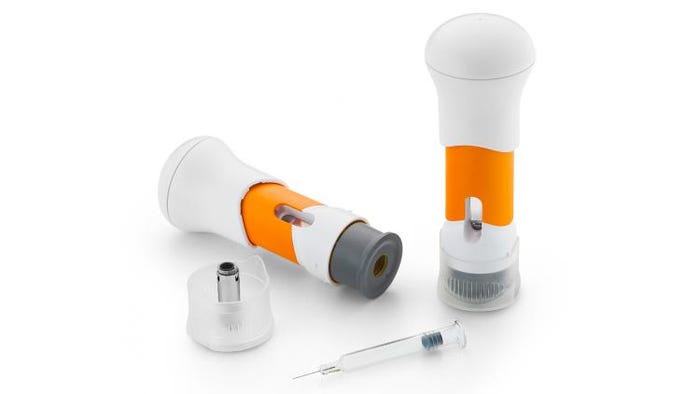
Silver Award: Methofill (methotrexate) Self Inject from Accord Healthcare Ltd.
Used for Methofill Self Inject, the handheld SelfDose injector from West Pharmaceutical Services helps patients self-administer a subcutaneous injection. The device is part of a combination product that is supplied to patients with a standard 1mL prefilled syringe already loaded inside. Its overall design makes it easy to use, at home, for people with limited dexterity—what’s called a “worse-case” patient. Being able to self-inject at home saves them from having to make what can be difficult trips to a clinical setting.
Contrary to a standard autoinjector, the SelfDose is a patient-controlled manual injector. This means the patient can adjust the delivery rate of the drug to what’s most comfortable for them. Why is that control important? Higher viscosity formulations—a characteristic of many of today’s fast-growing biologic drugs—may be painful when injected too quickly.
Additionally, the device is designed not to be scary, intimidating or “medical looking” to help patients be more comfortable using it. The intuitive and non-intimidating self-injection device helps ensure successful adherence with a window to confirm drug delivery, a color indicator of completed injection and an audible click confirming completed injection.
Instead of following the trend to smaller autoinjector devices, the SelfDose is deliberately large, with an easy-to-grip mushroom top. That gives patients more area to grasp, which is especially helpful when dexterity is low.
The product was tested with patients who were never able to self-inject before, and they were successful in a simulation during their first use of the SelfDose injector.
The design also allows pharmaceutical partners to insert their own drug in a prefilled syringe into the device, and any dose volume at any viscosity up to 1mL can be used without modifying the product.
NEXT: Gold Awards: Taltz Injection Devices from Eli Lilly and Co., and Surgicel Powder Absorbable Hemostat from Ethicon US LLC
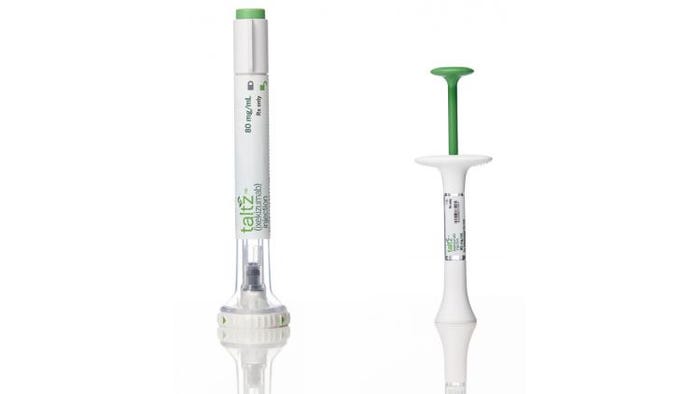
Gold Award: Taltz Injection Devices from Eli Lilly and Co., in collaboration with IDEO
The Taltz single-use injectors are designed to treat people with moderate to severe plaque psoriasis and psoriatic arthritis with Eli Lilly and Co.’s Taltz medication. The intuitive devices come in auto-injector and prefilled-syringe form, giving patients and caregivers a choice of an injection experience that best fits their needs.
The entry states: “During in-person, in-context interviews, we continuously heard, ‘I hate injections,’ with some patients requesting, ‘Help me do this better,’ and others saying, ‘Do it for me.’ This feedback revealed a need for two different products: an enhanced, prefilled syringe for the former user group, and an autoinjector for the latter. Both devices, however, make it possible for patients to self-inject and allow this to be a therapy anyone can self-administer at home.”
Patients and/or caregivers are more comfortable treating psoriasis at home, and that helps improve adherence. A simple aesthetic design with soft colors also helps people feel comfortable with the devices—storing them in the refrigerator, traveling with them and using them.
The designs go well beyond just beautiful graphics, to highly functional devices engineered with ergonomic features that ease the treatment process. The Taltz injectors give patients clear visual and auditory feedback throughout the injection process via simple icons, bright colors and clicks.
For the autoinjector, patients simply press the green trigger button, and the device regulates the dose, depth of needle insertion and rate of medicine delivery—without the user having to keep count of the time. After the dose has been delivered, an integrated mechanism retracts, locking the needled syringe in the autoinjector.
More than 94% of people first using it said the ergonomic Taltz autoinjector was easy to use and they were confident in their ability to use it. It also has soft, grippable material at all touchpoints and can be used with one hand. The autoinjector requires a low-force macro motion to activate, which can be done without any fine dexterity or pinch strength—traits people with psoriatic arthritis often lack. This flexibility in use means patients can inject Taltz in additional areas on their body, which allows for more comfortable site rotation between injections.
The second version—the manual injector—gives patients greater control over the injection process. An enlarged finger flange with grip-enhancing material and texture helps keep users’ fingers comfortable and steady as they press downwards to inject. The entry explains: “The plunger rod and syringe inside the device are closely guided, so that even if users’ hands happen to shake, the product continues to feel stable and reassuring, and neither the needle nor the rod wobble.”
The devices are easily grip-able due to their shape and texture. Plus the packages have an over-sized loop so arthritic patients can hook their finger into the loop. Minimal force or dexterity is need then to easily open the packaging.
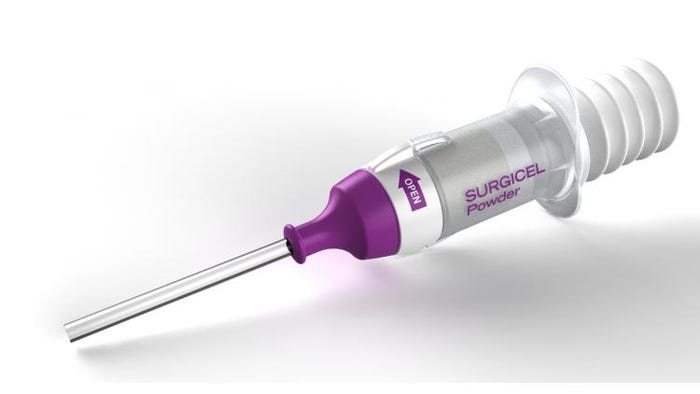
Gold Award: Surgicel Powder Absorbable Hemostat from Ethicon US LLC
Surgicel Powder Absorbable Hemostat stops continuous, broad-surface oozing and bleeding fast, even on friable or raw tissue when other conventional methods are impractical or ineffective. The unique structure of the powder penetrates the surface of the blood to get to the sources of bleeding and is also a proven bactericidal against dangerous hospital pathogens.
Made through a proprietary process, the oxidized regenerated cellulose (ORC) powder is ready to use out of the package, without any preparation.
Compared to competitive products, Surgicel can be easily applied and with more control. A chamber design on the device keeps the powder securely in place until ready to be dispensed. The device then controls how much powder is released per pump—regardless of device orientation—and ensures consistent coverage. According to Ethicon, Surgicel Powder dispenses a consistent amount of powder for about 15 pumps—again, regardless of device orientation.
Precise application enables the powder to penetrate pooling blood to stop bleeding at the source—and without having to apply pressure, unlike gauze or fabric. Nerve bundles and some tissues or organs are fragile or sensitive and can be at risk for damage from applying pressure.
The dual innovation—a new formulation that interacts smartly with blood and an applicator that delivers quick and easy coverage to bleeding surfaces—accelerates clotting and could potentially reduce surgical procedure time by as much as 25 minutes.
There could possibly be money savings, too. According to Ethicon, 32% to 68% of open surgeries experience a major bleeding event, while 30% of patients develop complications related to their bleeding event. By preventing or controlling these events, this delivery device can lower healthcare costs.
Ethicon reports that sales have surpassed expectations. From product launch Nov. 15, 2017, to mid-January 2018, sales were $1.5 million. And the company says feedback from users and sales representatives has been overwhelmingly positive.
********************************************************************************
Packaging solutions come to Minneapolis: As part of the region’s largest advanced design and manufacturing event, MinnPack 2018—and the five related shows taking place alongside it—brings 500+ suppliers, 5,000+ peers and 60+ hours of education together under one roof. Register for free today.
About the Author(s)
You May Also Like




