Patching in large volume doses
December 11, 2015

Patch injectors promise a level of patient comfort and convenience in the parenteral delivery of high-dose biologics.
Patch injectors are among new technologies in development for enabling self administration of large molecule and viscous biologics. In eliminating the need for multiple injections and infusions, these hands-free devices promote medication regimen compliance by making self-dosing easier for the patient.
“There are many biologics in development that are tending toward greater injection volumes—over 1 mL in the subcutaneous space—than have historically been seen in the marketplace. Many of these are applications where the patient will be injecting themselves,” says Megan Lan, senior product manager, BD Medical—Pharmaceutical Systems.
“With a hands-free patch injector system, the patient can take a large volume injection with just one needle stick, where you might need multiple needle sticks with a standard autoinjector, pen, or syringe. This gives the patient a more preferred experience,” Lan adds.
BD (Becton, Dickinson and Company; www.bd.com) is developing a patch injector system for hands-free automatic delivery of high volume dose or highly viscous drugs. BD Microinfuser Patch Injector Technology can deliver a wide range of therapeutic drugs to the subcutaneous tissue through a slow infusion.
The dose is delivered over a customizable period of time, based on the needs of the therapeutic drug and the needs of the patient population. With a patch injector, delivery can last for a few seconds to several hours, as the hands-free format helps patients endure longer injections.
The BD Microinfusor patch injector is a disposable mechanical device that requires no patient assembly. The needle is hidden before and after use. A passive needle safety feature engages after needle removal.
The BD Microinfusor patch injector is a drug-delivery system with ergonomic features built around a primary container of a prefillable syringe. Configured in a flat form, it is held tight against the skin via an adhesive patch. As a mechanical design without electronics, the device offers familiarity to the target population and ease of disposal and recycling, Lan says. “We have attempted to create a delivery system with a simple design as the market is seeking a simple solution for these large volume and or high viscous drug applications.”
Simple Mechanics
The patient peels off an adhesive liner, applies the device to the injection site, depresses a button, and waits for the injection to take place. The patch supports a preset bolus injection volume and injection rate. After injection, the device is removed and disposed of.
“Patients are looking for an intuitive system to take their therapies easily. With the BD Microinfusor patch injector there is no need to change the container—it is prefilled, preassembled, and disposable—and requires very few steps to use,” Lan says.
“From a pharmaceutical company perspective, companies want to ensure their drugs are protected. This is a simple mechanical system, which will not heat up the drug, with a well-characterized primary container,” she adds.
Needle size influences the patient experience with the device. While a larger bore needle will enable a faster flow, it may also engender an increased perception of pain. “Because we have deep expertise in needle design and manufacturing, we are able to customize the gauge and length of the needle to the needs of the therapy and patient to give them a more comfortable injection experience. Ultimately the goal is to help with adherence and compliance with drug therapy,” Lan says.
West (www.westpharma.com) has developed the Smart Dose Electronic Patch Injector System Platform Technology for these high volume dose applications. West acquired the rights to the technology after three years of collaborative development with an Israel-based patch injection system developer. With commercial scale up underway, the product is available for customers’ stability work and testing, says Graham Reynolds, vice president of marketing and innovation, West.
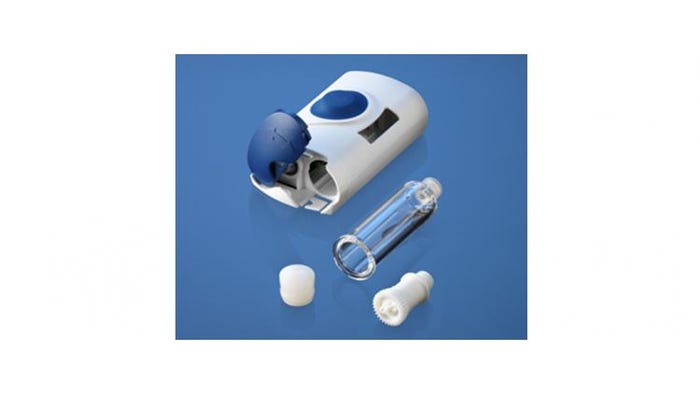
West's SmartDose Electronic Patch Injector System Platform features electronic pump control and programmable dosing.
The SmartDose system features electronic pump control and programmable dosing. The patient presses a button to activate the device and insert the needle. An electronic motor then drives the plunger forward to start the flow of the drug. A patented telescoping plunger rod drives the plunger forward at a controlled, preset rate. In programming, the manufacturer can set the rate of dose delivery. The device records how the dose was given, capturing dosing dates and flow rates, with wireless reporting capability.
The single-dose disposable unit features audio and visual signals to guide the patient.
The SmartDose system uses prefillable cartridges made of West’s Daikyo Crystal Zenith (cyclic olefin polymer), with FluroTec-coated components.
“We built the system around the CZ container because we believe the CZ plastic combined with our FluroTec-coated plungers is the better solution for biologic drugs. When you have a pump precisely driving a mechanism, the benefits of plastic become apparent—you have better dimensional tolerances and more consistent sliding forces of the plunger,” Reynolds says.
Biologics may be sensitive to silicon oil, tungsten, and adhesives used in glass syringes.
The cartridge format makes the patch injector device dimensionally shorter, Reynolds says, as the cartridge and device lay flat against the skin. Upon inserting the cartridge, a needle pierces the cartridge septum. The drug flows through a sterile fluid pathway to a second needle that goes into the skin at a 90-degree angle.
“Emerging biologic therapies may require a larger dose than the typical 1 mL dose given subcutaneously by a conventional syringe or auto injector. Some of these molecules by nature are very large, viscous, and concentrated. You can get to the effective dose by giving multiple shots of 1 mL; or the alternative is to deliver a larger dose slowly over a period of time with a patch injector,” Reynolds says.
Targeting The Home
Conventional single-use, disposable auto injectors are designed to ensure reliable and safe drug administration by patients and their caregivers in home settings.
The SelfDose injector platform technology, developed by Janssen Biotech Inc. in collaboration with West, features a novel drug delivery mechanism to this end.
The SelfDose injector relies on the patient’s hand force to insert the needle and deliver the dose. After a safety cap is removed and the device is applied to the injection site, the user pushes down on a broad knob with the flat of the palm to initiate and complete the injection. After injection, a passive safety system activates and protects the needle.
The SelfDose injector foregoes the typical system in autoinjector devices that rely on a spring to deliver the dose.
By eliminating this element, risk of incomplete injection and syringe breakage is reduced.
“Janssen Biotech recognized the need for an injection system designed around patient needs, particularly for those with limited dexterity. The SelfDose injector fits easily in the palm of the hand where people will have more pushing force,” says Reynolds.
“SelfDose relies on the patient’s own hand force to insert the needle and deliver the dose in one pushing motion. When the device is removed, a passive needle cover covers the needle completely—the patient never sees the needle,” Reynolds adds.
The patient can control the injection rate—the speed at which the dose is delivered—for a measure of control over injection pain. Patients reference a color indicator to verify that the dose was completely delivered.
With an integrated 1-mL-long pre-filled syringe as the container, the spring-less device is designed to simplify the manufacturing and regulatory approval process, and afford good economics.
After early-phase development by Janssen, West will commercialize the Self Dose injector as part of its portfolio of self-injection devices. “We are in the process of scaling up manufacturing and final development and product is available for customer testing,” Reynolds says.
The BD Physioject disposable autoinjector is a single-use, fixed-dose autoinjector designed for ease of use in a home care setting, with safety features that prevent unintended actuation, and needle stick after injection. The device was first launched with a drug product in 2010.
The BD Physioject is prequalified in multiple configurations so pharma customers have the flexibility to customize, making the development process simpler and faster, Lan says.
“Human Factors and our patient-centric design process is a very important focus area for our self-administration platforms. We perform multiple iterations of usability studies and other types of patient research as early as possible within the development process of any of our platforms. The focus of this is to make a product as easy to use and error free as possible when it is in the patients’ hands,” says Lan.
“We find also this data can also aid our pharma customers in their registration process, so they can focus their time on getting their drug product to market quickly,” she adds.
In the BD Physioject disposable autoinjector design process, patient studies revealed unanticipated results.
“In some of our early research, we found some patients with limited hand strength and dexterity experience difficulty in operating their current injection systems. We focused on patient interaction with the system by performing usability studies with many forces, shapes, and functionalities such as optimal cap removal forces and injection activation forces,” says Lan.
“A surprising finding was that a smaller product is not necessarily better. Ease of use and the ability of the patient to manipulate the device is rated higher than discretion. Using our understanding of patient needs, we designed the product to be easier to hold and use.
“Additionally, we found many patients want visual control confirming that they are taking their therapy correctly. They want to see the injection process. We are the only company to offer a 360° viewing window, giving patients the confidence that their drug is being administered correctly,” Lan says.
SIDEBAR:
Sunovion Nasal Spray Includes Dose Indicator
Zetonna Nasal Aerosol from Sunovion Pharmaceuticals Inc. will be available in the United States for this year’s allergy season after recent FDA approval.
Sunovion collaborated with Aptar Pharma (www.aptar.com) in the development of Zetonna, a pressurized metered-dose inhaler (pMDI) for delivering a corticosteroid indicated for treatment of seasonal and perennial allergic rhinitis symptoms.
Zetonna features Aptar’s Landmark dose indicator technology built into an innovative actuator for delivering the non-aqueous dry aerosol spray to the nostril.
“The aerosol generates a ‘dry’ mist as opposed to traditional ‘wet’ sprays delivered with a spray pump. The perception by the patient is different. Some patients prefer dry mists because they tend to minimize dripping and swallowing, which may be associated with an unpleasant taste,” says Pierre Carlotti, vice president, marketing and communication, Aptar Pharma Prescription Division.
Zetonna is the first use in a nasal application of the Landmark technology, a dose counting device that operates independently from the patient’s actuation force. Landmark counts down the doses, with color and numerical references indicating when the prescription needs to be refilled.
“The pMDI actuator is specifically designed according to human factor engineering and testing, with a dose indicator designed in such a way that it is robust, easy-to-access, and legible for the targeted population,” Carlotti says.
SIDEBAR:
Easing Inhalation
Tudorza Pressair from Forest Laboratories, Inc. offers novel features for patient ease-of-use and safety in long-term maintenance treatment of chronic pulmonary obstructive disease (COPD), such as chronic bronchitis and emphysema.
Recently FDA approved, Tudorza is a dry-powder multi-dose inhaler containing 60 metered doses for twice-daily delivery of aclidinium bromide inhalation powder, a long acting anticholinergic that was jointly developed by Forest and Almirall. The device is manufactured by Gerresheimer; Forest Ireland fills the formulation and completes device assembly, according to a Forest spokesperson.
Dry-powder inhalers are breath-actuated, relying on the force of patient inhalation to deliver the powder to the lungs. To support safe, efficient dosing, Tudorza features multi-sensory feedback through a colored control window that changes from green to red with an audible click on successful actuation of each dose. A safety mechanism reduces the potential for accidental double dosing. A lock out mechanism prevents the use of an empty container. In two Phase II studies in patients with moderate to severe COPD, Forest Labs found patients preferred its dry-powder inhaler design when compared with two pressurized metered-dose inhalers in commercial use. Patients rated the dry-powder inhaler as easier to use, easier to prepare the dose, and expressed an overall preference for the convenience of the Forest Labs device.
About the Author(s)
You May Also Like


