Could Mexico’s New Warning Labels Trigger Labeling Laws Elsewhere?
Required front-of-pack (FOP) declarations on all prepackaged food and non-alcoholic beverages sold in Mexico could inspire Latin American or other countries to establish their own labeling regulations as well.

In recent years, Latin American countries Chile (1), Peru (2), Brazil (3), Colombia (4), Argentina (5), and Ecuador (6) have established or considered establishing front-of-pack (FOP) labeling requirements for packaged foods and beverages. These laws vary in the style of communicating information, ranging from information-based quantitative declarations to warning sign-styles.
Mexico, which for several years has had an information-based FOP labeling system, recently adopted a warning sign-framework similar to FOP regulations in Chile — but that goes further.
Mexico’s new requirements impose unique warning messages for artificially sweetened products and foods containing added caffeine. Foods bearing the new FOP labels in Mexico will not be permitted to bear nutrition-related claims or use marketing that is appealing to children (such as using cartoon characters, celebrities, or interactive elements).
The FOP changes, in amendments to NOM-051-SCFI will be implemented in three stages starting on October 1, 2020. Some amendments have a compliance date of April 1, 2021.
In addition to the proliferation of FOP labeling systems throughout Latin America, international organizations, like the World Health Organization (7) and the United Nations’ Food and Agriculture Office (8), continue to urge countries to adopt similar front-of-pack labeling measures. As a result, it is possible that Mexico’s FOP amendments will have a domino effect in other Latin American countries and around the globe.
FOP warning symbols are intended to discourage intake of certain nutrients.
The amendment to the Official Mexican Standard titled, NOM-051-SCFI/SSA1-2010, Especificaciones Generales De Etiquetado Para Alimentos Y Bebidas No Alcohólicas Preenvasados-Información Comercial y Sanitaria (“General Specifications of Prepackaged Food and Non-Alcoholic Beverages — Commercial and Sanitary Information,” hereinafter “NOM-051, as amended”) sets forth new warning labeling requirements for all prepackaged food and non-alcoholic beverages sold within Mexico. Most notably, NOM-051, as amended, requires manufacturers to place octagonal warning symbols on certain consumer products that state, for example, “Excess Calories,” “Excess Saturated Fat,” “Excess Sugars,” and “Contains Caffeine — Avoid in Children.”
Clauses 4.5.3.4 to 4.5.3.4.7 mandate manufacturers to place octagonal shaped warning stamps on the FOP of foods and non-alcoholic beverages that have levels of sodium, trans fats (9), sugars, or saturated fats that exceed the thresholds shown in Table 1 (10).
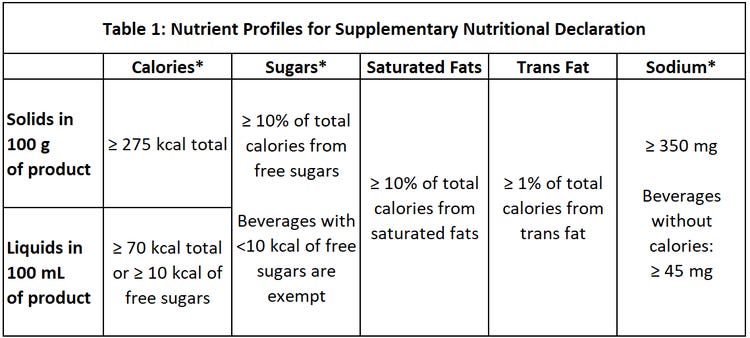
* During the second phase that starts on October 1, 2023, the thresholds will change as follows:
Calories: liquids in 100 mL of product will have a threshold of ≥ 70 kcal total or ≥ 8 kcal of free sugars;
Sugars: beverages with <10 kcal of free sugars will no longer be exempt;
Sodium: ≥ 1 mg sodium per kcal or ≥ 300 mg and for calorie-free beverages ≥ 45 mg.
The required FOP symbols are as follows:

Importantly, the warnings are based on levels of nutrients in 100 grams or milliliters, rather than a serving. Thus, even if a food has a very small serving size (such as chewing gum or a seasoning), warnings will be required if the product exceeds the thresholds on a 100g or ml basis.
Clause 4.5.3.4.2 of NOM-051, as amended, sets forth special conditions for those products whose main exhibition surface is shorter than 40 cm2 and 5 cm2. Such products will need to provide a single symbol to indicate the number of stamps that would have been on the product had it been of regular size. For example, the label of a small product that would have required two stamps (such as for calories and sugar) would be required to place this symbol on the principal display panel:

The Mexican Ministry of Health intends the new labeling requirements to limit the intake of “Critical Nutrients,” which are considered risk factors associated with chronic diseases when consumed in excess.
Requirements for artificially sweetened and caffeinated products are unique.
The amendment also added requirements under clauses 7.1.3 and 7.1.4 of NOM-051 that apply to products that contain sweeteners or caffeine, which are two substances that the Mexican Ministry of Health has determined can be harmful to children.
Under clause 7.1.3 of NOM-051, as amended, if a product’s ingredient list includes any sweeteners, the front-of-pack label must bear the following warning, meaning (“CONTAINS SWEETENERS, NOT RECOMMENDED FOR CHILDREN”) in all capital letters as shown below.

Interestingly, the Mexican authorities do not appear to see a conflict between the warning for artificial sweeteners and their clearances as additives under Mexico’s “Acuerdo por el que se determinan los aditivos y coadyuvantes en alimentos, bebidas y suplementos alimenticios, su uso y disposiciones sanitarias” (“Agreement that determines the processing aids and additives in foods, beverages, dietary supplements, their use and sanitary dispositions”).
Likewise, under Clause 7.1.4, if the prepackaged product contains added caffeine within the list of ingredients in any quantity, the front-of-pack label must bear the following warning statement, which translates to “CONTAINS CAFFEINE — AVOID IN CHILDREN,” in capital letters, as shown below.

Presumably, given that the warning only applies to products with caffeine added as an ingredient, the warning will not be required for foods that contain caffeine as a natural constituent, such as bottled coffees and teas.
The sweetener and caffeine warning statements must be placed on the upper right-hand side of the principal display panel and under any other seals that the product may be required to bear (11).
Notably, clause 4.1.4 of NOM-051, as amended, states that products that do not bear octagonal warning seals or warning statements will be allowed to include a statement saying, “Este producto no contiene sellos ni leyendas” (“This product does not contain warning seals or stamps”).
Nutrition claims and marketing that appeals to children is prohibited.
Pursuant to clause 6.3 of NOM-051, as amended, a prepackaged product cannot bear claims that relate to a nutrient that is the subject of an octagonal warning seal mentioned in clause 4.5.3.4.1 or a legend set forth in clauses 7.1.3 and 7.1.4.
As of April 1, 2021, prepackaged products that bear one or more FOP warning stamps or the sweetener warning statements must not include “children’s characters, animations, cartoons, celebrities, athletes or pets, interactive elements, such as visual-space games or digital downloads” that are directed at children to incite, promote, or encourage consumption of products with excess critical nutrients or sweeteners (12). However, products may include an endorsement of a product by an organization or association provided that the claim is substantiated by objective and reliable evidence and the product does not exceed one or more of the added critical nutrients established in Table 1 above (13).
Although some companies have argued that this change in the law directly affects the intellectual and industrial property rights protected and regulated in Mexico’s Industrial Property and Copyright Law, the Mexican government has moved forward with enforcing Mexico’s amendment to NOM-051.
NOM-051, as amended, has other significant requirements.
In addition to the new requirements discussed above, the Mexican government now mandates that prepackaged food and beverage products comply with the following requirements:
• Nutritional information is based on servings of 100 g/ml (14). While one could previously declare nutritional information based on 100 g or 100 mL or per serving/container basis, NOM-051 now requires food manufacturers to declare on a 100 g/100 mL basis. However, an additional declaration on a per serving or per container basis is permitted on a voluntary basis.
• Added sugars must be grouped and declared as the compound ingredient “azúcares añadidos” (“added sugars”) in the ingredient list (15).
• There have been some changes to allergen labeling requirements, including exceptions for certain fish and certain highly processed ingredients from fish and soy, and adding mollusks as a category (16).
• For imported products, companies may include the statement, “fabricado o envasado por o para” (“manufactured or packed by or for”) followed by the name and address, as appropriate (17).
Enforcement and state laws add further complexities.
NOM-051-SCFI/SSA1–2010, as amended, is entering into force in a staggered manner. The new requirements will be implemented in three stages. The first stage began on October 1, 2020, the second stage begins on October 1, 2023, and the third stage will start October 1, 2025 (18). As further discussed below, these thresholds will determine what products will be mandated to bear octagonal shape stamps and warning statements.
The Federal Consumer Protection Agency (“PROFECO”) and the Federal Commission for Protection against Sanitary Risk (“Comisión Federal para la Protección contra Riesgos Sanitarios” or “COFEPRIS”) will jointly monitor and enforce NOM-051, as amended (19). PROFECO has noted that the fines for non-compliance with front labeling requirements set forth in NOM-051, as amended, may reach 781,920 pesos, which is approximately US$38,800 (based on February 2, 2021, exchange rate) (20).
The new requirements at the federal level in Mexico dovetail with recently proposed or enacted laws in at least 24 states, including Mexico City, that impose further restrictions on processed foods. Several of these enacted or proposed laws prohibit minors from purchasing foods high in sugar and with high calorie content (21).
For example, the state of Oaxaca recently approved a modification to the current law titled, Bis de la Ley de los Derechos de Niñas, Niños y Adolescentes del Estado de Oaxaca (Law of the Rights of Girls, Boys and Adolescents of the State of Oaxaca), which places restrictions on the sale of “junk food” to children. Specifically, Oaxaca’s recent modification prohibits the “distribution, sale, gift and supply to minors, of sugary drinks and packaged foods of high caloric content in [Oaxaca] and in public and private educational institutions of basic and upper secondary education” (emphasis added).
The Oaxacan government’s modification also prohibits the inclusion of any of these products in “vending machines located in public and private educational institutions of basic and upper secondary education or commercial establishments.”
Other states, like Jalisco have also adopted similar legislation. As of December 1, 2020, the state of Jalisco will begin to sanction companies that fail to comply with the new standard on the labeling of prepackaged food and non-alcoholic beverages (22).
Impact on industry could be significant.
The new rules summarized above will force companies to either reformulate (23) products to avoid FOP warnings or rethink packaging. The new rules provided very little time for industry to comply.
The Mexican Council of the Consumer Products Industry (“ConMéxico”), an industry group that represents more than 40 major food and beverage producers, urged the Mexican government to postpone the effective date of NOM-051, as amended, but an extension was not provided. Under the terms of the law, companies can provide new FOP information using stickers until March 31, 2021, after which formal printed labels will be required.
The impacts of the law are already being felt, with some manufacturers having removed iconic imagery from its labels to comply with NOM-051, as amended (24). Thus far, legal challenges to the new requirements have not been successful, and industry is in the process of achieving compliance.
Citations:
1 - See “Manual de Etiquetado Nutricional de Alimentos.” Gobierno de Chile, available at http://www.indap.gob.cl/docs/default-source/default-document-library/manual-de-etiquetado-minsal-vf.pdf?sfvrsn=0 (last accessed January 26, 2021).
2 - See “Decreto Supremo Nº 017-2017-SA: Decreto Supremo que aprueba el Reglamento de la Ley Nº 30021, Ley de Promoción de la Alimentación Saludable.” Gobierno del Perú, available at
http://www2.congreso.gob.pe/sicr/cendocbib/con4_uibd.nsf/5289E04A2A160ABD052581A10070E6CE/$FILE/2_decreto_supre_017_de_alimentacion.pdf (last accessed January 26, 2021).
3 - See “Resolução de Directoria Colegiada: RDC Nº 429.” Diário Oficial Da União. October 8, 2020, available at https://www.in.gov.br/en/web/dou/-/resolucao-de-diretoria-colegiada-rdc-n-429-de-8-de-outubro-de-2020-282070599 (last accessed January 26, 2021); see also “Instrução Normativa-IN RDC Nº 75.” Diário Oficial Da União. October 8, 2020, available at https://www.in.gov.br/en/web/dou/-/instrucao-normativa-in-n-75-de-8-de-outubro-de-2020-282071143 (last accessed January 26, 2021).
4 - See “Colombia tendrá etiquetado nutricional en los alimentos envasados.” Ministerio de Salud y Protección Social. February 26, 2020, available at https://www.minsalud.gov.co/Paginas/Colombia-tendra-etiquetado-nutricional-en-los-alimentos-envasados.aspx#:~:text=para%20alimentos%20envasados-,El%20principal%20objetivo%20es%20que%20la%20informaci%C3%B3n%20nutricional%20que%20est%C3%A1,y%20comprensible%20para%20el%20consumidor.&text=El%20%C3%BAltimo%20paso%20en%20la,comenzar%C3%A1%20en%20noviembre%20de%202022 (last accessed January 26, 2021).
5 - See “Qué propone la Ley de Etiquetado Frontal de Alimentos que aprobó el Senado y cómo funcionó en otros países.” TN. October 26, 2020, available at
https://tn.com.ar/salud/nutricion/2020/10/29/que-propone-la-ley-de-etiquetado-frontal-de-alimentos-que-trata-el-senado-y-como-funciono-en-otros-paises/ (last accessed January 26, 2021); see also “Etiquetado Nutricional Frontal de Alimentos.” Secretaría de Gobierno de Salud. November 2018, available at https://www.fbioyf.unr.edu.ar/evirtual/pluginfile.php/174458/mod_resource/content/1/INFORME-etiquedato-nutricional-frontal-alimentos%202018.pdf (last accessed January 26, 2021).
6 - See “Reglamento Sanitario de Etiquetado de Alimentos Procesados para el Consumo Humano.” La Ministra de Salud Pública. 2013, available at https://www.controlsanitario.gob.ec/wp-content/uploads/downloads/2014/08/REGLAMENTO-SANITARIO-DE-ETIQUETADO-DE-ALIMENTOS-PROCESADOS-PARA-EL-CONSUMO-HUMANO-junio-2014.pdf (last accessed January 26, 2021).
7 - See “Guiding principles and framework manual for front-of-pack labelling for promoting healthy diets.” World Health Organization. 2019, available at
https://www.who.int/nutrition/publications/policies/guidingprinciples-labelling-promoting-healthydiet/en/ (last accessed January 26, 2021).
8 - See “Food labeling in Latin America and the Caribbean: interventionism or a necessary fight against malnutrition?” Food and Agriculture Organization of the United Nations. October 16, 2017, available at http://www.fao.org/in-action/agronoticias/detail/en/c/1044218/ (last accessed January 26, 2021).
9 - See Norma Oficial Mexicana NOM-051-SCFI/SSA1-2010. “Especificaciones generales de etiquetado para alimentos y bebidas no alcohólicas preenvasados-Información comercial y sanitaria, publicada el 5 de abril de 2010.” Diario Oficial. March 27, 2020, available at http://dof.gob.mx/2020/SEECO/NOM_051.pdf (last accessed January 26, 2021) (Clause 4.5.3.4.2 of NOM-051, as amended sets forth special conditions for those products whose main exhibition surface is shorter than 40 cm2 and 5 cm2).
10 - See Norma Oficial Mexicana NOM-051-SCFI/SSA1-2010. “Especificaciones generales de etiquetado para alimentos y bebidas no alcohólicas preenvasados-Información comercial y sanitaria, publicada el 5 de abril de 2010.” Diario Oficial. March 27, 2020, available at http://dof.gob.mx/2020/SEECO/NOM_051.pdf (last accessed January 26, 2021) (Clause 4.5.2.3 of NOM-051, as amended, notes the products that are exempt from including the nutrition declaration. Exempt products include products that include a single ingredient, herbs, spices, among others.)
11 - See Norma Oficial Mexicana NOM-051-SCFI/SSA1-2010. “Especificaciones generales de etiquetado para alimentos y bebidas no alcohólicas preenvasados-Información comercial y sanitaria, publicada el 5 de abril de 2010.” Diario Oficial. Clause 4.5.3.4.7 of NOM-051, as amended. March 27, 2020, available at http://dof.gob.mx/2020/SEECO/NOM_051.pdf (last accessed January 26, 2021).
12 - See Id. Clause 4.1.5 of NOM-051, as amended.
13 - See Id. Clause 4.1.4 of NOM-051, as amended.
14 - See Id. Clause 4.2.2.1.8 of NOM-051, as amended.
15 - See Id. Clause 4.2.2.2.3 of NOM-051, as amended.
16 - See Id. Clause 4.2.4.1 of NOM-051, as amended.
17 - See Id. “Articulos Transitorios.”
18 - See “Update on Revised NOM-051 Labeling Requirements.” USDA Foreign Agricultural Service. October 18, 2010, available at https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Update%20on%20Revised%20NOM-051%20Labeling%20Requirements_Mexico%20ATO_Mexico_10-18-2010.pdf (last accessed January 26, 2021); see also “La Modificación a la NOM-051 entrará en vigor el 1 de octubre de 2020.” Gobierno de México. August 1, 2020, available at https://www.gob.mx/salud/prensa/comunicado-conjunto-economia-salud-cofepris-y-profeco?tab= (last accessed January 26, 2021).
19 - See “Update on Revised NOM-051 Labeling Requirements.” USDA Foreign Agricultural Service. October 18, 2010, available at https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Update%20on%20Revised%20NOM-051%20Labeling%20Requirements_Mexico%20ATO_Mexico_10-18-2010.pdf (last accessed January 26, 2021); see also “La Modificación a la NOM-051 entrará en vigor el 1 de octubre de 2020.” Gobierno de México. August 1, 2020, available at https://www.gob.mx/salud/prensa/comunicado-conjunto-economia-salud-cofepris-y-profeco?tab= (last accessed January 26, 2021).
20 - See de la Rosa, Eduardo. Ochoa, Cristina. “Lo que debes saber sobre el nuevo etiquetado de alimentos.” Milenio 2020. October 1, 2020, available at
https://www.milenio.com/negocios/nuevo-etiquetado-frontal-de-alimentos-nom-051 (last accessed January 26, 2021); see also Ley de Infraestructura de la Calidad. Accessed November 18, 2020, available at http://www.diputados.gob.mx/LeyesBiblio/pdf/LICal_010720.pdf (last accessed January 26, 2021) (Penalties are not discussed in NOM-051, but are discussed in other Mexican laws, such as Article 155 of Mexico’s Ley de Infraestructura de la Calidad).
21 - See GACETA Parlamentaria No. 366. August 19, 2020, available at https://www.congresocdmx.gob.mx/media/documentos/d4bc11d3a31daf32d1402ab3463170eefee69a00.pdf (last accessed January 26, 2021); see also Prensa. “Aprueba LXIII Legislatura eliminar venta y distribución de comida chatarra y bebidas azucaradas a menores de edad.” August 17, 2020, available at https://congresotabasco.gob.mx/boletin/aprueba-lxiii-legislatura-eliminar-venta-y-distribucion-de-comida-chatarra-y-bebidas-azucaradas-a-menores-de-edad/ (last accessed January 26, 2021); Caporal, Issac. “Diputados michoacanos van contra comida chitarra.” El Sol de Zamora. August 26, 2020, available at https://www.elsoldezamora.com.mx/local/diputados-michoacanos-van-contra-comida-chatarra-5672947.html (last accessed January 26, 2021); Perez Alfonso, Jorge A. “Prohibida en Oaxaca, venta de alimentos chatarra a menores.” La Jornada. August 6, 2020, available at https://www.jornada.com.mx/ultimas/estados/2020/08/06/prohibida-en-oaxaca-venta-de-alimentos-chatarra-a-menores-9122.html (last accessed January 26, 2021).
22 - See López, Isaura. “Sancionarán a empresas que incumplan con la nueva norma de etiquetado.” El Occidental. October 4, 2020, available at https://www.eloccidental.com.mx/local/noticias-nueva-norma-de-etiquetado-5844333.html (last accessed January 26, 2021).
23 - See Gómez Mena, Carolina. “Reformulan leche de Liconsa para evitar etiquetado frontal.” La Jornada. October 18, 2020, available at https://www.jornada.com.mx/ultimas/economia/2020/10/18/reformulan-leche-de-liconsa-para-adecuarla-a-nom-051-8629.html (last accessed January 26, 2021).
24 - See “¿Por qué desaparecieron los enanitos del chocolate Costanzo?” La Orquestra. October 26, 2020, available at https://laorquesta.mx/por-que-desaparecieron-los-enanitos-del-chocolate-costanzo/ (last accessed January 26, 2021).
About the Author(s)
You May Also Like






