Own it: How more effective packaging approvals can help you avoid a recall
October 5, 2015
Jackie Leslie
Accountability for packaging graphics approvals becomes diluted as the number of people involved in the review process rises. Prevent this “Bystander Effect” by sharing the responsibility within a team—but with clear assignments and authority.
More than 19 million food units were recalled in Q1 of 2015, according to Stericycle Inc. The FDA’s website shows the majority of these recalls were due to missing ingredients or undeclared allergens on packaging, meaning the pack was approved but some critical, possibly life-saving information was missing. And the products were recalled.
As a result of recall, a brand’s reputation for quality gets damaged and all the materials, money, time and effort that went into getting the product and package produced and on the shelf are wasted.
Why does this happen?
Sometimes, simple mistakes such as proofreading, typos and omissions occur because everyone thinks there is power in the sheer number of approvers. It’s easy to think:
“Surely if we have eight sets of eyes on this package, between all of us we will find every error and correct it.”
Even though this logic is widespread and seems sound, it is incorrect, ineffective and potentially dangerous.
Let’s play it out. Consider these thoughts that may occur in a typical group of eight packaging approvers.
Approver 1: “Looks good as-is. And if I’m wrong, one of the other approvers after me will catch any mistakes.”
Approver 2: “The last person didn’t catch anything, so I’ll quickly look it over, but she’s smart, I’m sure it’s fine. And if I’m wrong, one of the approvers after me will discover any issues.”
For Approvers 7 and 8, the thinking can easily become:
“By now the other approvers must have found everything, so I probably can’t contribute anything. And if I find something now, we’ll have to start all over again. They can’t all be wrong, and the deadline is tomorrow!”
Even though it is common to think this way, this is a recipe for disaster, especially when rushed or under pressure.
This behavior is similar to what Professor Robert Cialdini identifies as the Bystander Effect, which is what happens when a group of people perceive that they are each bystanders and therefore not directly accountable for what they are experiencing. He contends that accountability is diluted because of the number of people involved. As a result, no one assumes true, full accountability for any portion of the activity. That means that the more people there are in the approver group, the less accountable any one of them feels for the approval.
We also know from experience that holding groups of individuals generally accountable for entire deliverables, such as packaging artwork, is not as effective as holding individuals specifically accountable for discreet deliverables, such as specific components of the package.
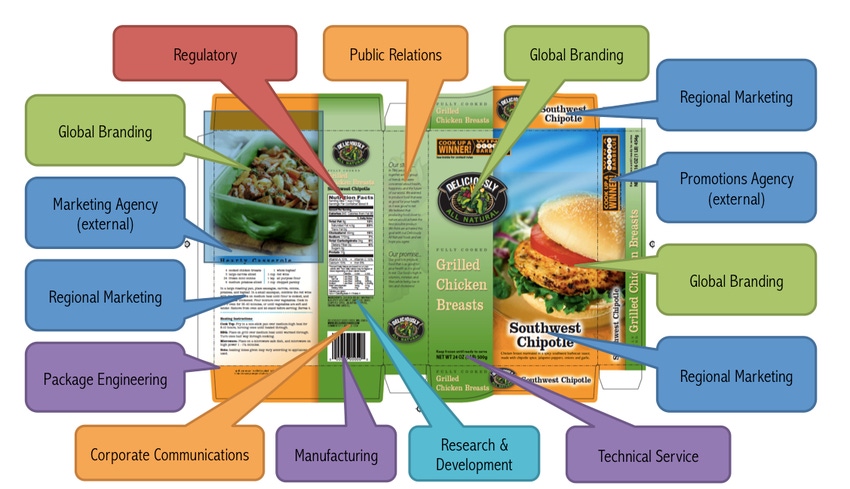
Our staff recently conducted a workshop with a client and one of the exercises we facilitated was an “every square inch” review, in which we looked at every inch of the package to identify each piece of content and assign ownership. Our client approached her team members, pointing to a portion of the packaging, and asked: “Who owns this? How can I be certain this information is correct?” In some cases, her team members did not know. No one took responsibility for owning certain content. In another instance, multiple people claimed ownership for the same single piece. Getting roles and responsibilities right is absolutely critical.
There’s a better way to manage your package and artwork development process. Workflow and proofing technology help package development teams organize the complexity of processes in which many people need to approve artwork and packaging, making the process efficient, effective and repeatable while mitigating risk. Make sure your workflow engine allows simultaneous approvers access from any computer or mobile device.
For example, in a digital workflow, the artwork or packaging manager can assign approval authority to multiple specialists: a person in R&D for ingredients, another in Regulatory for the facts panel and one in Marketing for claims and address information, for example. Great workflow and proofing systems clearly show each person what is assigned to them for comment and records those comments, thereby upholding the integrity of the information.
To get the most out of decision makers, make sure to provide clear review instructions and make available relevant source documentation (such as specifications, technical drawings and approved regulatory content). These essential resources should be simple to find and accessible during the review process. Many times reviewers are not sure what to check due to lack of context, but this is easily remedied by guidance documents that detail who is requesting the change, who is approving the request and what documentation is available to support the change.
Using technology simplifies and provides valuable context to approval workflows. It helps users automatically compare the new statement to the old statement, approved graphic to final graphic, new version to previous version. Add the power to approve once and then execute approved changes across multiple stock-keeping units (SKUs) and now teams are achieving quality results at scale.
All of this makes team members more effective by motivating them to own their decisions. This drives the kind of accountability that regulated industries demand from product manufacturers. A software that can enable these capabilities is essential to your defense against audit and recall risk.
The new thinking for each approver can be: “This is my portion, and only I can prove the right answer to it. I am certain it’s right and confident approving.”

Jackie Leslie, project management professional (PMP), is director of process consulting at BLUE Software LLC. Based in Toronto, Leslie works with companies that are exploring ways to gain better control over their brand content. With a background in project management and consulting, coupled with 15 years focused on brand management, Leslie identifies process improvement opportunities, shares best practices and recommends technology-based solutions to support getting products to market faster with higher quality information.
You May Also Like


