4 Observations from Pharmapack Europe 2024
Industry experts Mindy Katz and Mathias Romacker from the professional service organization Kymanox share their insights from what they saw and heard in Paris.
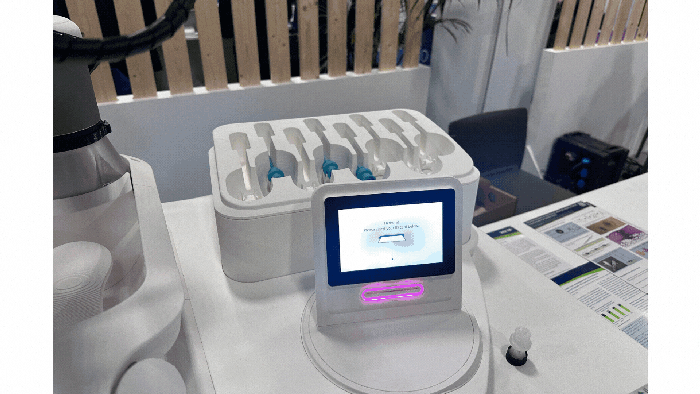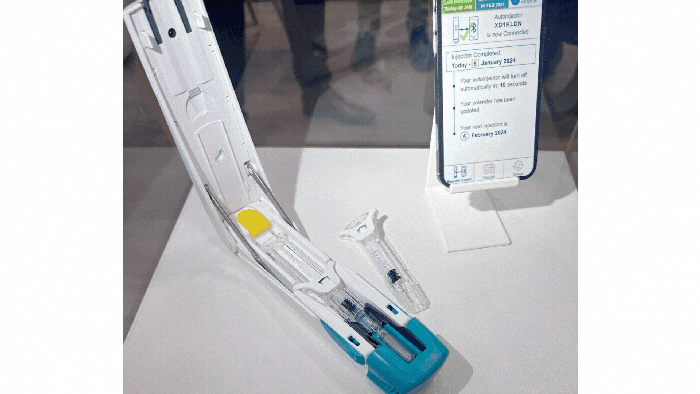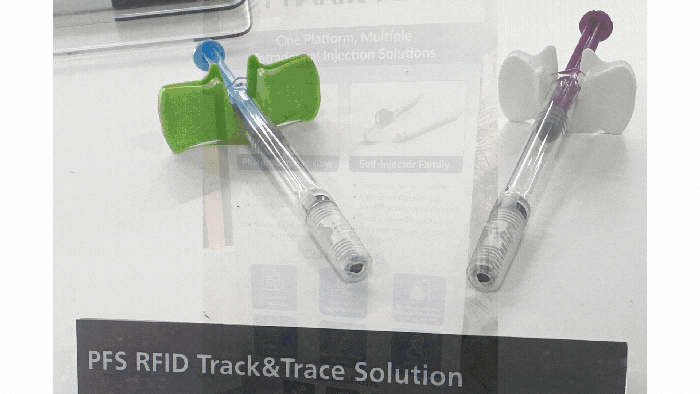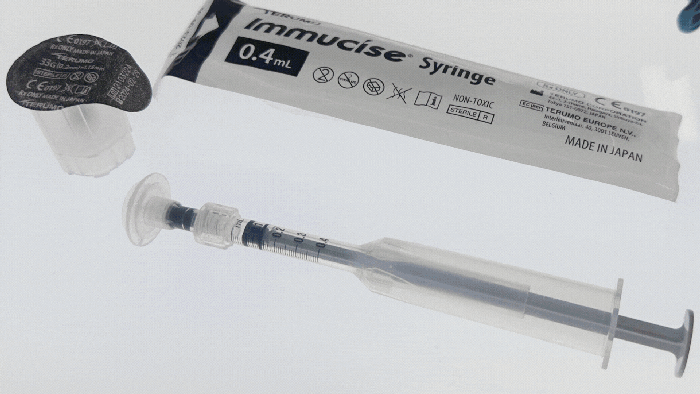
At a Glance
- Sustainability from various stakeholder’s perspectives
- Debates about the necessity of certain drug-delivery device categories and patient preferences
- Flurry of new products, from prefilled syringes to reusable on-body injectors
Nearly all the major players in pharmaceutical packaging and combination products attended Pharmapack Europe 2024, which took place in Paris on January 24-25. Well-established for decades, the event draws several thousands of visitors to the exhibition, workshops, lectures, presentations, and panel discussions.
Pharmapack is an important conference for the pharmaceutical packaging industry, and as such reflects the ecosystem in terms of trends, challenges, and topics of particular importance. Here are four insights derived at this “it” event for pharmaceuticals and combination devices.
1. Sustainability shows its significance.
In recent years, Pharmapack has placed significant emphasis on sustainability. Not only have conference organizers introduced multiple initiatives to reduce waste, recycle, reuse, choose sustainable materials, and reduce meat consumption, but conference content also has a significant focus on sustainability. Multiple lectures and workshops promoted and discussed sustainability in the pharmaceutical industry from various stakeholder’s perspectives.
At Pharmapack 2024, three distinctly different types of companies stood out in terms of sustainability:
• Companies with products inherently “sustainable” by nature, such as fully mechanical drug-delivery devices (without electronics) or semi-reusable devices (where the electronic component is reusable). While sustainability may not have been the leading factor in making these design choices, these companies are now leveraging the device’s characteristics as an additional selling point from a strategic marketing perspective, as sustainability gains more focus in the eyes of pharmaceutical customers.
• Companies with products considered to have a higher environmental impact, such as fully disposable components/devices or those containing electronic components. These companies face the challenge of quantifying their environmental impact, comparing it to others, and highlighting other areas of sustainability, such as environmental management systems and environmentally conscious supply chain strategies. Such companies often engage in third-party device lifecycle assessments, for an objective quantification of their product’s environmental impact, while simultaneously highlighting other product advantages such as usability, cost, and manufacturability.
• Startups and early-stage companies whose primary focus is sustainability. Sustainability seems to be their main selling point and primary driver for business decisions and design choices. In such a competitive market, it remains to be seen if sustainability as the leading strategy can be sufficient to secure partnerships with pharmaceutical companies.
Individuals and pharmaceutical companies have highlighted the importance of corporate environmental policies in recent years. It is important to balance different parameters, ensuring that essential considerations, such as access to products, successful delivery of medication, as well as environmental impact, are all addressed.

Examples of drug-delivery devices seen at Pharmapack Europe 2024. KYMANOX
2. Categorization of drug-delivery devices.
Conversations at Pharmapack delved into the borders between different drug-delivery devices: prefilled syringes, auto-injectors, on-body delivery systems, near-body delivery systems, and infusion pumps (see photos of examples above). Discussions centered around hard and soft limits (such as volume limitations and the number of injections) and what parameters determine transitions between device categories. Industry professionals debated the necessity of certain device categories and considered patient preferences in device design.
3. News and new products.
Several noteworthy updates emerged from Pharmapack 2024:
• New labels from Schreiner MediPharm are designed to improve barrier properties against oxygen ingress in COC plastic prefilled syringes (see photo 1 in the slideshow).
• Cosmetic companies are collaborating with pharmaceutical packaging producers to design packaging with a “more medical look,” as shared by Hoffmann-Neopac.
• More injection devices, previously disposable, now offer reusable versions. Sonceboz showcased samples of wearable on-body injectors with reusable features (see photo 2 in the slideshow).
• Credence Medsystems presented an intriguing idea for a prefilled syringe with a staked needle offering needle length choices for vaccine administration (see photo 3 in the slideshow).
• Multiple companies discussed the use of recycled plastics in their products, such as TekniPlex’s blister pack using 30% recycled plastics (see photo 4 in the slideshow) and Bormioli’s rPET bottles featuring “virgin quality” polymers.
• Given the deep-freeze requirements for the first generation of mRNA vaccines, Schott is offering prefilled syringes that can be solidly frozen (see photo 5 in the slideshow).
• Ypsomed and Schott have a cooperation to manufacture 5ml prefilled syringes used in Ypsomed auto-injectors (see photo 6 in the slideshow). At the exhibition, we learned that other device manufacturers like SHL Medical also offer prefilled syringe-based disposable auto-injectors.
• Owen Mumford showcased its UniSafe Safety Syringe prefilled syringe (see photo 7 in the slideshow) and its reusable connected fully mechanical auto-injector for UniSafe 1ml.
4. The importance of face-to-face meetings.
Despite the prevalence of webinars, Zoom meetings, and Teams communication, Pharmapack served as a reminder of the invaluable significance of face-to-face interactions. Holding products in hand provided a different perspective than viewing them online, and interactions with manufacturers revealed insights not easily gleaned from press releases. Informal discussions over wine and hummus sparked innovative ideas that might not have surfaced via email or virtual meetings. The value of “water cooler conversations” became evident, emphasizing the importance of meeting industry colleagues in person and engaging in great conversations during panels and networking opportunities.
Altogether, Pharmapack provided industry professionals an opportunity to review shifts in manufacturing and design trends for drug-delivery systems and key touchpoints for attendees to connect with fellow colleagues. As the year progresses, we will track how these trends play out and what new and current ones evolve to improve safety, compliance, cost, and sustainability.
About the Author(s)
You May Also Like






