Electronic nasal-spray package limits dosing for opioid medication
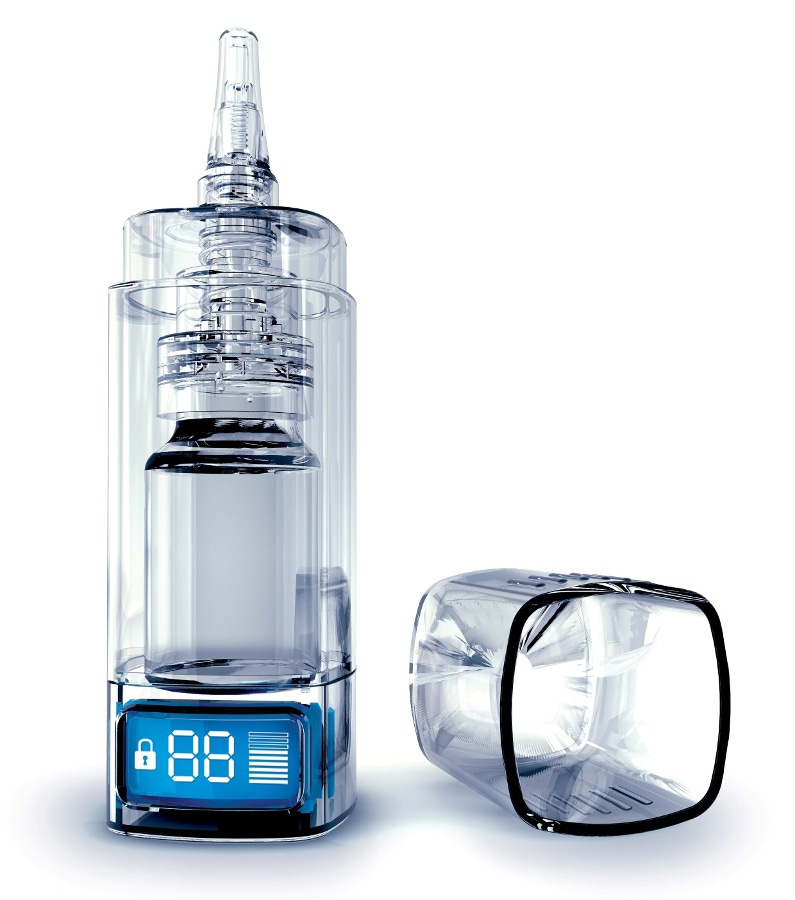
Patient compliance is important with any prescription drug, but particularly if the drug could be habit-forming. To help ensure compliance with Instanyl, a nasal opioid, Takeda Pharmaceuticals International AG has chosen pharmaceutical packaging that doesn’t just count the doses—it electronically limits the number of doses that can be delivered in a 24-hour period.
Takeda will be using the e-Lockout device from Aptar Pharma (a unit of AptarGroup Inc.) to package a new version of Instanyl, a nasal spray used to treat breakthrough pain in adult cancer patients. The packaged product, Instanyl DoseGuard, will launch in Europe, which is also where the e-Lockout device is manufactured. It will be available in various multidose strengths; the e-Lockout device can be configured for 10, 20 or 40 doses.
Instanyl DoseGuard features a mechanism that temporarily locks usage after a defined number of spray actuations. Patients can take two doses per episode of breakthrough pain; after the second dosage within an hour, the device automatically locks. The product can be used for up to four breakthrough pain episodes daily.
The device’s electronic display keeps track of priming actuations and remaining doses, and it also displays whether the device is locked or available for use. When the device locks itself, the display starts a visual countdown to let the patient know when it will unlock.
Daniel Usureau, vp-sales in the EMEA Prescription Div. of Aptar Pharma, explains how the e-Lockout device works: “The electronic component controls the unlocking of the mechanism. The locking is done mechanically.”
The package consists of opaque plastic injection-molded components, electronic module and metered dose pump. It comes with a child-resistant cap that is removed by pressing on both sides of the cap, turning the cap counter-clockwise and lifting it off.
Earlier this year, the European Medicines Agency (EMA) authorized the marketing of Instanyl DoseGuard. According to Aptar Pharma, the e-Lockout is the first fully integrated, electronic nasal lockout device to gain EMA approval.
************************************************************************
Learn about the latest developments in pharmaceutical and medical packaging at EastPack 2017 (June 13-15; New York City) and the co-located MD&M East show. Register today!
About the Author(s)
You May Also Like




