8 potential UDI code readability issues and the critical role of vision
September 30, 2016

September 2016 brings several key deadlines for Unique Device Identification (UDI), a system of medical device identification rules issued by FDA in 2013. UDI requirements state that the device labeler, in most cases the manufacturer, must include on medical devices a device identifier (DI) that identifies the labeler and the specific version or model of device and a production identifier (PI) that includes one or more of the following: lot or batch number, serial number of a specific device, expiration date of the device, and the date the device was manufactured.
As medical device manufacturers work toward compliance, they are encountering serious challenges, particularly in reading UDI codes. This article will identify 8 types of readability issues manufacturers may encounter and explain how sophisticated vision technology could help address them.

Above: The UDI consists of a device identifier and a production identifier.
THE RULES
While Class III medical devices have been required to carry UDI labeling since September 2014, by September 24, 2016, these Class III devices must carry UDI as a permanent mark on the device itself if that device is intended to be used more than once and is intended to be reprocessed before use. In addition, all Class II devices must now bear UDI on their labels and packages, and the dates on these labels must meet formatting rules (implantable, life-saving, and life-sustaining Class I and II devices have had to carry UDI labeling since September 2015).
By September 2018, all Class I devices must carry UDI labeling, and Class II devices that require DPM must carry the UDI on the device. Finally, Class I devices that require DPM must carry a permanent UDI on the device by September 2020. Click here for more details on the rules.
There have been some recent extensions for devices packaged together and for combination products, but significant numbers of medical devices must now comply with UDI requirements.
Click to the next page to learn about 8 Potential UDI code readability issues
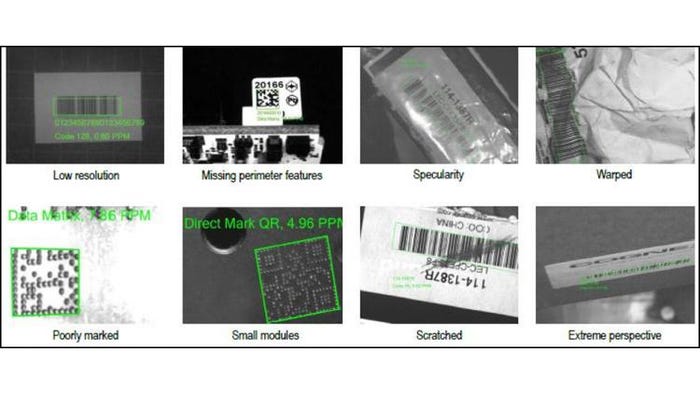
8 Potential UDI code readability issues
UDIs are typically marked as a bar code, a QR code, or a DataMatrix code. Reading 1-D and 2-D UDI codes and text can be challenging in real-world applications because of the wide range of surfaces on which the codes may be marked, variations in the size, shape, position and orientation of the code, the potential for degradation in printing and marking of the codes, and variations in ambient lighting.
The task is even harder for medical devices that don’t have much room for a code, such as scalpels or syringes. Laser etching onto metals is the typical approach, but it’s not easy. The laser must be calibrated correctly, and the code must be marked clearly enough to be read.
Following are 8 potential UDI code readability issues:
1. Low resolution. Many times parts or codes on one line can change in size, which means that a standard resolution bar code reader can read some codes, but not others. Look for readers that offer subpixel processing and reading capabilities.
2. Missing perimeter features. Sometimes, codes are printed too close to an edge, causing the code to be missing key finder patterns. Look for readers that employ algorithms that can accommodate codes without a quiet zone or clocking or finder pattern.
3. Specularity. Specular reflection occurs often when codes are printed on glossy materials. These can be very hard for laser scanners because glare could occur through the laser line. Image-based readers just need a small sliver of the code in order to decode.
4. Warped. Warped codes can sometimes seem as though they are missing part of the code. Look for systems with algorithms that can read codes that do not have quiet zones or clocking or finder patterns.
5. Poorly marked. Poorly marked codes could be due to a printer being low on ink and having poor contrast for example. Powerful algorithms can read the most damaged codes.
6. Small modules. For very small codes, a variety of lensing options can compensate and read the code.
7. Scratched. Look for systems that only need a small portion of a 1-D code to read it, so if a code is marked with a marker or scratched it can still be read.
8. Extreme perspective. Part or code placement can be difficult, and the code can sometimes be presented to the reader at an extreme angle. Look for systems that can accommodate such angles.
Recent technology advancements are benefitting medical device manufacturers and other supply chain contributors rushing to comply with UDI regulations. For example, in the vision-based ID reader space, software algorithms have been developed that are able to find and decode even damaged and poorly marked 1-D or 2-D codes through a wide range of illumination, marking, and material variations. Another advancement involves application-specific solutions for vision systems that eliminate the need for programming and pre-built documentation that substantially reduces the time required for FDA validation.
TAKING ACTION
While UDI offers potential advantages in securing and improving the efficiency of the medical device supply chain, medical device manufacturers are encountering serious challenges to compliance.
FDA may fine-tune the regulations in the future. In the meantime, milestones are occurring on a regular basis. For instance, the number of products for which direct marking is required has increased. So it’s incumbent upon device manufacturers to make plans and take action to ensure compliance.

Kasey Tipping (above) serves as Technical Marketing Specialist - ID Products for Cognex Corp. (Natick, MA).
For more information, contact Cognex at One Vision Drive, Natick, MA 01760-2059 USA. Tel (Toll Free): 1-877-COGNEX1 (1-877-264-6391), Fax: +1 508 650-3344, Email: [email protected], Web: http://www.cognex.com/
About the Author(s)
You May Also Like


