New Pharmaceutical Packaging Test Method Earns USP Approval
Now accepted by the United States Pharmacopeia, a new test method measures water-vapor transmission rate in pharmaceutical blisters with more consistent results than the current desiccant method.
November 19, 2020
The United States Pharmacopeia’s Packaging and Distribution Expert Committee recently revised General Chapter <671> Containers-Performance Testing (Ref 1) to add an alternate method for the determination of water-vapor transmission rate (WVTR) of single-unit and unit-dose container closure systems, such as blisters. This alternate method — using water instead of desiccant — was vetted by a leading packaging development laboratory through a rigorous comparison to the unchallenged desiccant method, the standard since the 1970s.
The science of this gravimetric method is not complex — but should not be over-simplified. The alternate method uses the same science based upon the vapor pressure differences created by controlled humidity across a permeable film, such as that of a blister cavity. Relative humidity (RH) and vapor pressure are used to express the environmental chamber conditions and the headspace inside the test containers (blisters).
WVTR can be measured in terms of desiccant weight gain — or water weight loss — for a container closure system held in an environment with constant temperature and RH. Test containers filled with water provide a constant vapor-pressure difference across test container walls, offering improvements in WVTR testing compared to desiccant-filled containers where the vapor pressure inside may neither start nor remain at 0% RH.
Also, use of water-filled containers mitigates many of the challenges inherent in the preparation and handling of desiccant and desiccant-filled containers.
Vapor pressure difference.
In the traditional desiccant method, the difference in relative humidity (RH) or vapor pressure starts at 75% by loading the blister cavities of samples with desiccant, thus creating an RH near 0%, and storing the samples in an environmental chamber maintained at 75% (see Figure 1 below).
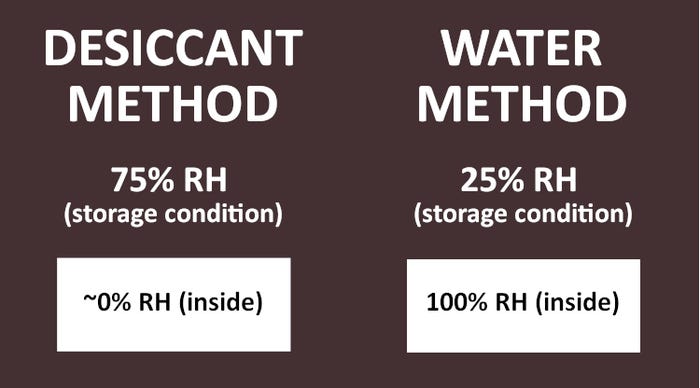
Conversely, the same but constant 75% difference in vapor pressure is created by loading the blister cavities of samples with water and storing the samples in an environmental chamber maintained at 25%. In both methods, the vapor pressure difference results in the migration (transmission) of water vapor across the film at a constant rate after steady state is achieved. This results in measurable weight change of the sample. For desiccant-filled blisters, weight gain occurs; for water-filled blisters, weight loss occurs.
With high-barrier blisters, equivalent results have been achieved with both methods over the USP prescribed study period of 29 to 35 days. However, due to the rapid exhaustion of desiccant capacity of low-barrier blisters, the USP desiccant method prescribes a single weight determination after storage for seven days. This line-through-the-origin (initial sample weight) does not allow one to determine a meaningful slope: the permeation rate.
Multiple weight determinations for low-barrier blisters are possible for the water method — because an adequate loading of water is possible, which maintains a constant vapor pressure difference longer than seven days. This allows for an adequate number of sample weights for a meaningful linear regression analysis. It has been demonstrated that the prescribed desiccant method for low-barrier blisters conducted at 25°C vs. 45°C reduces the permeation rate and desiccant exhaustion, thereby providing for more than one measurement and a linear regression analysis.
Use of desiccants can be problematic, however, because an inadequate amount of desiccant or incompletely dried desiccant may result in variability of the vapor-pressure differences between the inside and outside of the container during the study. This potential variability of the vapor-pressure difference can both bias and increase variability in WVTR determinations.
Reliability of results.
According to the traditional WVTR methods described in chapter <671> Containers-Performance Testing, test containers are to be filled with sufficient anhydrous desiccant to maintain an inside RH close to 0% during the study. Desiccant-filled test containers are held in an environmental chamber, and weight determinations are made over time.
Under constant environmental conditions, plastic [such as polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polyethylene terephthalate gly (PETG), and polychlorotrifluoroethylene (PCTFE)] container walls reach a steady state of water concentration after a preconditioning period. The inside RH remains constant during testing if adequate anhydrous desiccant is present. The weight gain of the desiccant is determined at this steady-state condition and is translated into a permeation rate for the test container closure system.
To obtain reliable WVTR determinations, the vapor pressure difference must remain constant inside and outside the test container throughout the study. Results from desiccant-filled test containers do not account for potential variability introduced if the internal RH of test containers neither starts at 0%, nor remains at 0% during the study.
USP General Chapter <1671> (Ref 2) states: “Desiccant methods are designed to maintain the internal RH below 10% throughout the study.” However, this internal RH range (0% to 10%) translates to variability in the internal RH; hence, variability in the results. In contrast, a test container filled with water will maintain a constant RH (100%) from the beginning to the end of the WVTR study, thereby reducing potential variability in the results, notwithstanding an inaccurate WVTR determination may be the final outcome.
To address the challenges involved in the use of desiccant-filled test containers, one can use the water-filled container method as an alternative approach for the determination of WVTR of container closure systems for solid oral drug products. Desiccant-filled test containers stored in a 40°C/75% RH chamber achieve a vapor-pressure difference of 65% to 75% if the internal RH created by the desiccant ranges from 0% to 10%. On the other hand, water-filled test containers stored in an environmental chamber at 40°C/25% RH achieve and maintain a constant vapor pressure difference of 75%.
Benefits of the water method.
In practice, multiple benefits can be realized by implementing WVTR testing using water as the test medium for blister samples.
With water, the variability of the fill amount does not result in variability of internal RH, and no interaction with container walls of common packaging materials (PE, PVC, PP, and PETG) has been observed. The stability of the internal RH afforded by water allows holding the samples or reusing them at a later time, or in multiple studies (such as different storage conditions using the same samples). Additional measurements or extensions of studies are possible without concern over depleting the water.
The uncertainty of having adequate desiccant which will maintain an inside RH near 0% (in a 75% RH chamber) is eliminated by creating a constant inside RH of 100% with water (in a 25% RH chamber).
Easier sample preparation.
Typically, those involved with preparing samples for WVTR testing will be skeptical of filling blisters with water. But filling with desiccant is not without challenges, including maintaining an adequate, dry supply; physical damage to foil lidding caused by the desiccant; the need for an oven to dry the desiccant (for example molecular sieves) up to 250°C; and potential exposure of the desiccant to moisture during filling and handling before testing. These challenges are eliminated with the water-filled container method.
This includes elimination of special handling requirements to protect desiccant-filled blister samples from excessive exposure to moisture before testing.

Also, samples filled with aqueous dye can be used for leak testing before or after WVTR testing. Use of dye enhances visual detection of gross leaks during the study.
Sample preparation techniques are simple for filling blisters with water. The best practice for delivering water into the cavities on the packaging line was developed using a manual syringe filled with water, allowing delivery of a few drops of water to each cavity without causing sealing problems. The surface tension of the water prevents spillage and splashing during line vibrations.
One to 10 drops of water are adequate for all blister types at 40°C/25% RH. For determining the WVTR of multiple-unit container closure systems, a 10% fill volume of water will create and maintain 100% RH during one or multiple studies.
Closing argument.
Water-filled test containers stored in an environmental chamber at 40°C/25% relative humidity (RH) maintain constant vapor pressure differences of 75%. Desiccant-filled test containers stored in an environmental chamber at 40°C/75% RH may not maintain constant vapor pressure differences of 75%. To maintain constant vapor pressure differences of 75% throughout the study, the water activity (aw) of the desiccant must remain very close to zero. The advantages of using water in place of desiccant are numerous. Make the change today.
One additional note: The advantages of using water in place of desiccant were not as significant for multiple-unit container closure systems as for single-unit blister container closure systems, with the most significant advantage being the elimination of desiccant (such as factors associated with increased cost and time). An alternate method for liquid-filled container closure systems developed in a business partner’s test lab has been submitted to the USP Expert Committee. USP’s Principle Scientific Liaison, Desmond Hunt, Ph.D., responded, stating the proposal is slated for consideration as an addition to USP <671> during the 2020-2025 Committee cycle.
For comments, collaboration, and questions, please contact Dwain L. Sparks, via email at [email protected].
References*
United States Pharmacopeia and National Formulary (USP 43-NF 38). Rockville, MD: United States Pharmacopeial Convention; 2020 https://online.uspnf.com/uspnf/document/1_GUID-99FB391E-ADC7-4247-B254-094C4DC7486C_3_en-US?source=TOC#C671S6. Accessed October 11, 2020 (subscription required).
United States Pharmacopeia and National Formulary (USP 43-NF 38). Rockville, MD: United States Pharmacopeial Convention; 2020 https://online.uspnf.com/uspnf/document/1_GUID-52690118-4600-4C92-B755-8E9CFFAE5B46_2_en-US?source=Search%20Results&highlight=1671. Accessed October 11, 2020 (subscription required).
*Note: Published changes to USP General Chapters <671> and <1671> become effective December 1, 2020.
About the Author(s)
You May Also Like




